After the cows enter the perinatal period, the qualified farmers need to set up a special delivery room for cows. The production bed should be dry, ventilated and quiet. However, when one thinks of setting up a delivery room for perinatal dairy cows, it will inevitably waste some manpower and material resources, so most of the breeding friends will give up. The friend who uses the golden baby dry fermented bed has no concern in this respect, because the golden baby dry spreader bed is not only usually used to save worry and effort, the key moment can also be a change, becoming the most intimate and comfortable cows delivery room.
Dairy cow perinatal period refers to the period of time before and after the cow's delivery. It includes late pregnancy and early lactation. As the cows have undergone major changes in the physiological aspects of this stage, the resistance has decreased, and it is very easy to become ill and may even affect the milk production throughout the lactation period. Therefore, it is very important to strengthen the feeding and management of dairy cows during the perinatal period. The Golden Baby Dry Sausage Fermenting Bed is very helpful in maintaining the cleanliness of the cow's delivery room and improving the cow's resistance. First of all, the fermentation bed promptly disposes of the excreted urine discharged from the milk steak, cuts off the spread of pathogens from the source, and guarantees the health and safety of the dairy cows for the post-natal and post-natal environment. Secondly, the fermented mattress material contains a large amount of beneficial bacterial protein, and after being eaten by cows, it can enhance the body immunity of the cow and increase the disease resistance. It ensures that the cows in the perinatal period rarely get sick or even get sick, and the medication is reduced, which saves the cost of breeding.
It is not difficult to understand that the delivery room for cows during the perinatal period also requires drying and ventilation. The dark, moist and immobile air is not only a breeding ground for dairy cows but also a taboo for all livestock breeding and is extremely unfavorable to the health of livestock. The dry-sweeping fermentation bed, as the name implies, has been effectively improved on the basis of the wet-type fermentation bed, and it does not need to be preliminarily fermented by water during the start-up process, and the dry material is also required to be dry during the operation. Therefore, in the contradiction between warmth and ventilation, the Tycoon Dry Fermenter bed emphasizes the importance of ventilation.
In addition, the insulation of the perinatal delivery room of the cows in winter is equally important. The ginba dry fermentation bed is maintained at about 20 degrees throughout the year, which is an excellent guarantee for the production environment of dairy cows. It should also be noted that pre- and post-natal postpartum dairy cows need adequate exercise to prepare for the delivery of lochia and postpartum lochia, and the practice of staying in the delivery room is not worth promoting. Details can be consulted: Beijing Huaxia Kangyuan Technology Co., Ltd. Telephone toll-free hotline Website: Taobao Website: http://shop35396982.taobao.com
Recommended reading
1. Fermented bed pigs can effectively prevent pig diarrhea
Http://?id=440&classid=153
2. Fertility in pigs can increase sow fertility
Http://?id=444&classid=153
Intermediates of Cladribine, Carvedilol, Lurasidone, olmesartan, Risedronate Sodium, Atazanavir, Saxagliptin, Dabigatran,Dapoxetine,Cefixime,Ceftaroline fosamil and etc.
In the short span of time, we have emerged as most promising pharmaceutical intermediates manufacturers, chemical intermediates and bulk drug intermediates suppliers. Our consistent supply, quality products and dedication towards clients have opened up many international avenues for our growth.
In addition, the company also can follow the customer's product needs custom synthesis services
MAIN API PRODUCTS USP/BP
|
PRODUCT NAME |
CAS NUMBER |
SPEVIFICATION |
|
Azithromycin |
117772-70-0 |
BEP |
|
Cefpirome Sulphate sterile |
84957-29-9 |
USP JP16 |
|
Ceftriaxone Sodium (Sterile) |
104376-79-6 |
USP31 |
|
Cefotaxime |
64485-93-4 |
USP30 |
|
Ciprofloxacin HCL |
85721-33-1 |
USP/BP |
|
Gentamicin sulphate |
1405-41-0 |
BP |
|
Levofloxacin |
100986-85-4 |
USP27 |
|
Lincomycin Hydrochloride |
859-18-7 |
EP6.0 |
|
Moxifloxacin Hydrochloride |
186826-86-8 |
USP31 |
|
Tigecycline |
220620-09-7 |
USP |
|
Linezolid |
165800-03-3 |
EP |
|
Dexamethasone |
50-02-2 |
USP/BP/EP |
|
Methylprednisolone |
83-43-2 |
USP/BP/EP |
|
Dexketoprofen trometamol |
156604-79-4 |
BP2008 |
|
Ibuprofen |
15687-27-1 |
BP |
|
Metamizol |
68-89-3 |
DAB |
|
Sulindac |
38194-50-2 |
USP/BP/EP |
|
Naproxcinod |
163133-43-5 |
USP28 |
|
Tripelennamine Hydrochloride |
154-69-8 |
USP28 |
|
Itraconazole |
84625-61-6 |
USP/BP |
|
Cytarabine |
147-94-4 |
USP31 |
|
Leucovorin Calcium |
1492-18-8 |
USP32 |
|
Valsartan |
137862-53-4 |
USP30 |
|
Telmisartan |
144701-48-4 |
USP31 |
|
Rosuvastatin Calcium |
147098-20-2 |
USP/BP |
|
Pitavastatin Calcium |
147526-32-7 |
USP/BP |
|
Fluvastatin |
93957-54-1 |
USP31 |
|
Vinpocetine |
42971-09-5 |
EP6.0 |
|
Atazanavir |
198904-31-3 |
BP |
|
Rosiglitazone |
122320-73-4 |
USP30 |
|
Esomeprazole Magnesium |
161973-10-0 |
USP/BP |
|
Topiramate |
97240-79-4 |
USP31 |
|
Fexofenadine HCl |
153439-40-8 |
Inhouse |
|
Bosentan |
147536-97-8 |
Inhouse |
|
D-Cysteine |
921-01-7 |
Inhouse |
|
D-Phenylalanine |
673-06-3 |
Inhouse |
|
Linagliptin |
668270-12-0 |
Inhouse |
|
Rivaroxaban |
366789-02-8 |
USP |
|
Saxagliptin |
361442-04-8 |
USP |
|
Vildagliptin |
274901-16-5 |
USP |
Major Pharmaceutical Intermediates
| Items Descripation | Structure | Application |
|
MICA ESTER CAS No: 246035-38-1 Purity: ≥98% |
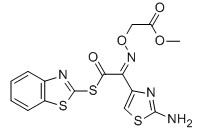 |
For Cefixime |
|
EHATA CAS No: 64485-82-1 Purity: ≥98% |
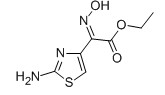 |
For Ceftazidine |
|
2-Chloroadenine CAS No: 1839-18-5 |
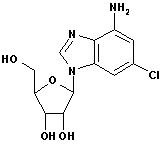 |
For Cladribine, Fludarabine et al |
|
Bicyclo(2,2,1)Heptane-2,3-di-exo-carboximide CAS No: 14805o-29-9 |
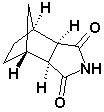 |
For Lurasidne |
|
(R,R)-1,2-Bis(methanesulfonyloxy methyl)Cyclohexane CAS No: 186204-35-3 |
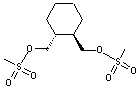 |
For Lurasidone |
|
3-(Piperazin-1-yl)benzol[d] isothiazole CAS No: 87691-87-0 |
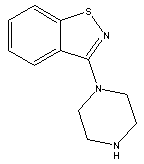 |
For Lurasidone |
|
Trityl olmesartan CAS No: 144690-92-6 Purity: ≥98% |
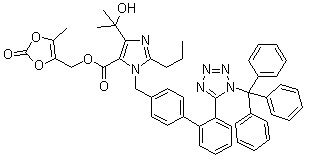
|
For olmesartan |
|
3-Acetyl Pyridine CAS No: 350-03-8 |
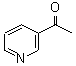
|
For Risedronate Sodium |
|
3-(AceticAcid)pyridine HCL CAS No: 6419-36-9 |
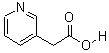 |
For Risedronate Sodium |
|
Risedronic Acid CAS No: 105462-24-6 |
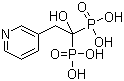 |
For Risedronate Sodium |
|
3-Hydroxy-1-adamantyl-D-Glycine CAS No: 709031-29-8 |
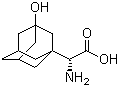 |
For Saxagliptin |
|
(1s,3s,5s)-3-(aminocarbonyl)-2-azabicyclo(3,1,0) hexane-2-carboxylic acid tert-butyl ester CAS No: 361440-67-7 |
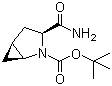 |
For Saxagliptin |
|
(S)-N-Boc-3- hydroxy-adamantylglycine CAS No: 361442-00-4 |
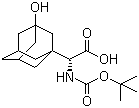 |
For Saxagliptin |
|
2-Azabicyclo[3.1.0] hexane-3-carbonitrile, (1s,3s,5s)- CAS No: 866083-42-3 |
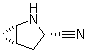 |
For Saxagliptin |
|
Ethyl 3-(pyridin-2-ylamino) propanoate CAS No: 103041-38-9 |
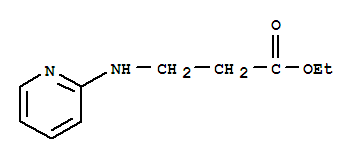 |
For Dabigatran |
|
N-(4-Cyanophenyl) glycine CAS No: 42288-26-6 |
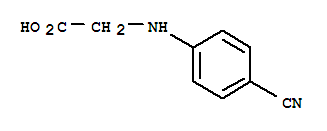 |
For Dabigatran |
|
4-methylamino-3-nitrobenzoic Acid CAS No: 41263-74-5 |
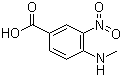 |
For Dabigatran |
|
S-3-Amino-3-phenylpropanoic acid ethyl ester HCL CAS No: 167834-24-4 |
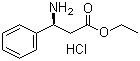 |
For Dapoxetine |
|
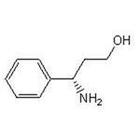
(S)-3-Amino-3-Phemylpropan -1-ol CAS No: 82769-76-4 |
|
For Dapoxetine |
|
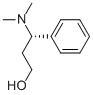
(S)-3-Dimethylamino-3-Phemylpropanol CAS No: 82769-75-3 |
|
For Dapoxetine |
|
4-{4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-butynil}-α,α-dimethyl benzene acetic acid CAS No: 832088-68-3 |
For Fexofenadine HCl | |
|
Methyl 2-(4-(4-chlorobutanoyl)phenyl)-2-methylpropanoate CAS No:154477-54-0 |
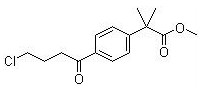
|
For Fexofenadine HCl |
|
5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone CAS No 461432-22-4 |
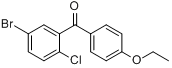
|
For Dapagliflozin |
|
4-(5-Bromo-2-chlorobenzyl)phenyl ethyl ether CAS No :461432-23-5 |
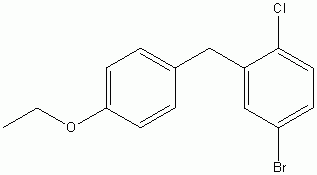
|
For Dapagliflozin |
Mica Ester,Pharma Intermediates,Ciprofloxacin Hcl Uses,Active Pharmaceutical Ingredients
NINGBO VOICE BIOCHEMIC CO. LTD , https://www.pharma-voice.com