Cell-free protein expression technology refers to the use of cell lysates containing components necessary for protein synthesis (ribosomes, transport RNA, aminoacyl synthase, start/extension/terminator, guanosine triphosphate, ATP, Mg2+ and K+). Protein synthesis was performed in vitro.
Cell-free protein expression systems have unique advantages over traditional bacterial or eukaryotic-based protein expression systems, including time savings and improved overall yield of functional, soluble, full-length proteins. In addition, cell-free protein expression systems are more suitable for the expression of toxic proteins such as kinases, and can also be labeled with modified tRNAs, incorporating non-natural amino acids at a specific site. At the same time, these systems can also be used for high-throughput experiments.
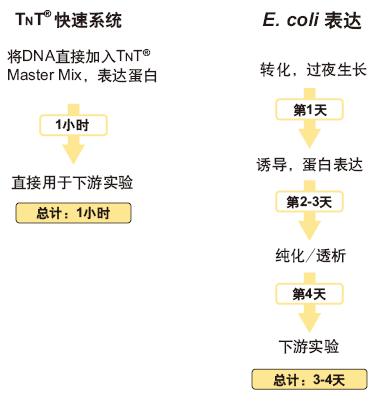
Rapid access to the protein of interest, from days to hours
The selection of a cell-free protein expression system, the most important considerations include the source of the cell extract or lysate, the template, and the desired protein yield to select a cell-free protein expression system suitable for the experimental system and downstream applications.
(a) template
When using an insert or vector for eukaryotic cell system protein expression, there are several factors to consider: (i) the ATG initiation codon should be the first ATG codon downstream of the transcription start site; (ii) Ideally, after the promoter, the ATG should be contained within the Kozak consensus sequence; (iii) the stop codon should be included at the 3' end of the template sequence; (iv) the stop codon should be followed by a synthetic Poly A tail (2). In addition, when using the TNT® T7 Coupled Wheat Germ System , the vector should also contain a T7 terminator sequence or the vector is linearized.
In prokaryotic systems, the choice of the start codon almost invariably depends on the presence of a ribosomal binding site (RBS), which contains the initiation signal of the reading frame. The optimized ribosome binding site can greatly increase the expression of proteins in prokaryotic cells. The prokaryotic system does not recognize any ATGs located upstream of the ATG start codon unless these ATGs contain a ribosome binding site in place.
When using rabbit Reticulocyte Lysate (RLL), DNA-mediated protein synthesis has the advantage of mRNA as the starting protein synthesis: when high-level protein synthesis is performed, there is no need to process mRNA. The complicated operation process.
(2) Translation based on eukaryotic RNA
Between 1950 and 1960, the researchers confirmed that rabbit reticulocyte lysates can be manipulated for exogenous mRNA-mediated protein synthesis, so that only the protein of interest can be synthesized. Both nuclease-treated rabbit reticulocyte lysates and Flexi® rabbit reticulocyte lysates added additives to optimize the translation process of mRNA. These additives include hemin - activation of elF-2α kinase (HRI) to prevent heme regulation; an energy generation system containing tested phosphocreatine kinase and creatine phosphate; and calf liver tRNA - Used to balance the consumption of tRNA species, which optimizes the use of codons and expands the range of mRNAs that can be efficiently translated. Compared to nuclease-treated rabbit reticulocyte lysates, the Flexi® rabbit reticulocyte lysate system provides greater flexibility to optimize many parameters of the translation reaction, including Mg2+ concentration, K+ concentration. And the existence of DTT.
Wheat Germ Extract (WGE) contains the cellular components (tRNA, ribosomes, initiation factors, elongation factors, and termination factors) required for the synthesis of proteins. The extract system was further optimized: an energy generation system consisting of phosphocreatine and phosphocreatine kinase was added; spermidine was added to enhance the efficiency of protein chain elongation to prevent premature termination of the protein chain Occurs; magnesium acetate is added and its concentration is suitable for translation of most germline mRNAs. Finally, potassium acetate is also provided separately to optimize more types of mRNA.
The wheat germ extract is suitable for expressing small molecules of proteins or for expressing proteins rich in rabbit reticulocyte lysates. This system is also useful when the RNA preparation contains a small amount of dsRNA or thiol, which has the effect of inhibiting translation. When expressing plant proteins, yeast proteins or other fungal proteins, the researchers also found that wheat germ extracts are more suitable than rabbit reticulocyte lysates.
(3) Transcription and translation based on eukaryotic DNA
In the 1990s, a coupled transcription/translation (TNT®) system was developed that contained rabbit reticulocyte lysate or wheat germ extract with T7, T3 or SP6 RNA polymerase. These systems make DNA-based protein synthesis a reality.
The TNT® Coupled Reticulocyte Lysate Transcription/Translation Systems and the TNT® Quick Coupled Transcription/Translation Systems require only one tube. That is, the protein can be transcribed and translated using a plasmid as a template. The different components of the conventional TNT® coupling system are provided in separate packages, including a mixture of three amino acids: a mixture of methionine deletions, a mixture of cysteine ​​deletions or a mixture of leucine deletions. The TNT® Rapid Coupling System provides a mixed mother liquor that contains all of the reaction components (including the methionine-depleted amino acid mixture), reducing the loading step and saving time. The TNT® T7 PCR DNA Rapid System ( TNT® Quick for PCR DNA ) is specifically designed for linear DNA templates generated by PCR reactions. Such templates often require higher potassium and magnesium ion concentrations than plasmid DNA templates.
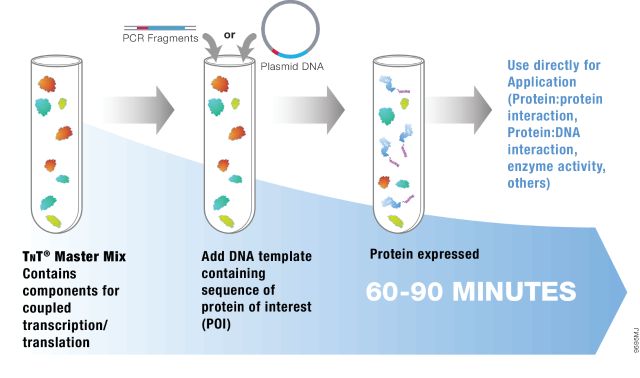
Brief illustration of TNT® Rapid System Application
For eukaryotic transcription/translational coupling reactions, in addition to rabbit reticulocyte lysates, the TNT® Coupled Wheat Germ Extract Systems is another option, which is also the response pattern of individual tubes. . Common wheat germ extracts typically use the SP6, T3 or T7 RNA polymerase promoter to synthesize RNA in vitro and then perform a translation reaction. In contrast, the TNT® wheat germ extract system integrates the transcription reaction directly into the translation reaction mixture.
(4) Transcription and translation based on prokaryotic DNA
The E. coli S30 Extract Systems (E. coli S30 Extract Systems) was prepared from the E. coli B strain in which the omp T intracellular protease and lon protease activity were absent. This deletion can greatly improve the stability of the expressed protein, otherwise the protein expressed in the cell will be degraded by the protease. The E. coli S30 extract system is capable of expressing a greater amount of protein, and when expressed in cells, these proteins are expressed in low amounts due to activation of the host-encoded inhibitor. The DNA template of the E. coli S30 extract may be linear or circular. The linear DNA-based S30 extract ( E. coli S30 Extract System for Linear Templates ) was prepared from the E. coli B strain, and the strain exc-cutase V (recBCD enzyme) was deleted. The activity of S30 extract with linear DNA as template is higher than that of S30 extract ( E.coli S30 Extract System for Circular DNA ) and T7 S30 Extract (For E. coli T7 S30 Extract System for Circular DNA ) low. When using these systems, the researchers only need to prepare cloned DNA with the appropriate prokaryotic promoter and ribosome binding sites.
Promega cell-free protein expression system classification
Eukaryotic cell-free protein expression system:
å…” Rabbit reticulocyte lysate system
昆虫 Insect Cell Extract System
麦 wheat germ extract system
Prokaryotic cell-free protein expression system:
å¤§è‚ æ†èŒ E. coli extract system
Pvc X-Ray Thread,Operation Pvc X-Ray Thread,Medical Pvc X-Ray Thread,Surgical Operation X-Ray Thread
Shaoxing Weijian Medical Supplies Co.,Ltd. , https://www.wetourmedical.com