〠Background 】
DC is the abbreviation of "Dendritic Cells", which is called "dendritic cells" in Chinese. It is named for its many dendritic or pseudo-protrusions. The DC was discovered in 1973 by the 2011 Nobel Prize winner and Canadian scientist Ralph M. Steinman. It is the most powerful antigen-presenting cell (APC) found today. DC has been shown to be the only APC that significantly stimulates the proliferation of naïve T cells, while other types of APCs (such as monocyte macrophages, B cells, etc.) can only stimulate activated or memory T cells. Therefore, DC is the initiator of the body's adaptive T cell immune response and plays an extremely important role in tumor immunity.
[ Culture principle ]
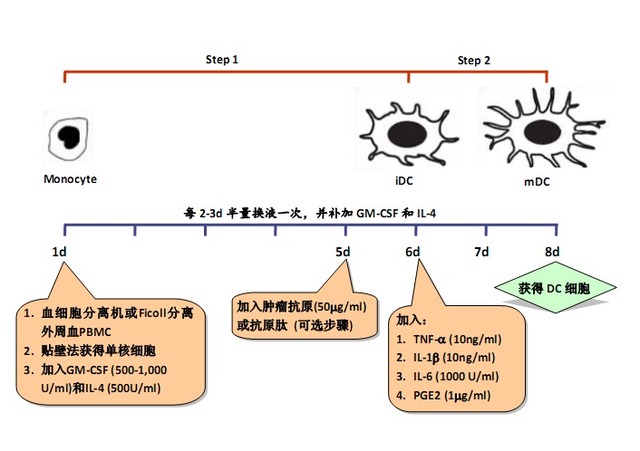
Because DCs need to be differentiated from other cell types and can be divided into different stages of maturation, they are usually divided into two steps:
Step 1: Inducing differentiation from DC progenitor cells (such as monocytes) to immature DC (imature DC);
Step 2: Inducing differentiation from iDC to mature DC (mature DC, mDC).
Step 1:
GM-CSF (granulocyte macrophage colony-stimulating factor):
GM-CSF is a hematopoietic growth factor that stimulates the formation of neutrophils and macrophage colonies in vitro and has the function of promoting the proliferation and development of early red megakaryocytes and eosinophils. GM-CSF was one of the first cytokines identified to have an effect on DC culture.
The function of GM-CSF in DC culture is to promote the differentiation of monocytes into large macrophage-like cells, and the expression of MHC class II molecules on the cell surface is enhanced, thereby enhancing the antigen presentation function of cells. In addition, GM-CSF can also promote the survival of DC.
IL-4 (Interleukin-4)
The role of IL-4 in the induction of DCs by monocytes is to inhibit the overgrowth of macrophages, thereby directing monocytes to differentiate into DCs. If IL-4 is not added to the culture system, monocytes will differentiate into macrophages. At the same time, IL-4 also has the ability to reduce the expression of CD14 molecules on the cell surface. Decreased expression of CD14 is an important marker for the differentiation of monocytes into DCs.
The combination of GM-CSF and IL-4 can differentiate mononuclear cells into immature DCs. At this time, DCs have strong antigen uptake and processing ability, but antigen presentation ability is weak. MHC class I, class II molecules and B7 family molecules (CD80, CD86, etc.) are moderately expressed on the cell surface, but CD14 is not expressed or expressed low.
Step 2:
TNF-α/IL-1β/ IL-6 (tumor necrosis factor-α/interleukin-1β/interleukin-6)
TNF-α, IL-1β and IL-6 are pro-inflammatory factors that are induced at the local and tumor sites. In vitro studies have shown that these three cytokines can down-regulate the macrocytosis of immature DCs and the expression of surface Fc receptors, so that the intracellular MHC class II compartments disappear, but can up-regulate cell surface MHC The expression of class I, class II molecules and B7 family molecules (CD80, CD86, etc.) differentiates immature DCs into mature DCs. At this time, the antigen uptake and processing ability of DCs is significantly weakened, and antigen presentation ability is significantly enhanced. Strongly activate T cells.
The TNF-α/IL-1β/IL-6 three-factor combination can induce complete maturation of DC in the absence of bovine serum culture conditions, thereby preparing DCs that can be applied to the clinic.
PGE2 (prostaglandin E2, prostaglandin E2)
PGE2 is also an inflammatory mediator and can be induced by inflammatory signals such as TNF-α, IL-1 and LPS (lipopolysaccharide). in
The addition of PGE2 to the TNF-α/IL-1β/IL-6 mature induction combination can further increase DC yield, maturity, migration ability and immune activation ability.
Of particular importance here is the increase in DC migration capabilities. Because TNF-α/IL-1β/IL-6 induces weak DC migration, it does not reach the lymph nodes and activate T cells. The addition of PGE2 induces mature DCs to migrate to lymph nodes more easily due to the high expression of surface chemokine receptors, which causes the body to fight against tumors.
Therefore, the combination of TNF-α/IL-1β/IL-6/PGE2 (see table below) is widely used in clinical practice and is considered the “gold standard†for the preparation of mature DCs.
| Component | Working concentration |
| TNF-a | 10ng/ml |
| IL-1β | 10ng/ml |
| IL-6 | 1000 U/ml |
| PGE2 | 1mg/ml |
〠Notes 】
1. Source of DC:
There are many sources of DC, including peripheral blood mononuclear cells (Monocyte), cord blood CD34+ cells, bone marrow and fetal liver, etc., but because peripheral blood mononuclear cells are the most accessible and the largest, they are widely used as DCs in clinic. Source of cells.
2. DC maturity:
We know that DC has immature and mature states, iDC and mDC, and the antigen presentation ability of DC is closely related to its maturation status.
iDC has a strong antigen uptake and processing capacity, but its antigen presentation ability is weak. When cultured in vitro, iDCs remove cytokines (ie, GM-CSF and IL-4) and reverse them to macrophages, and even under the maintenance of cytokines, the function of immature DCs is not to activate T cells, but Inhibits proliferation of T cells.
mDC, on the other hand, has a weak antigen uptake and processing capacity but a strong antigen-presenting ability. Thus, T cells can be activated to cause an immune response. Moreover, even after the DC is matured, even if the cytokine is removed in the culture system, the state and function of the DC can be maintained without reversal.
Therefore, it can be seen that DC must be fully mature before it can be used for immunotherapy.
3. DC-free bovine serum culture:
DCs were originally obtained in culture systems containing Fetal Calf Serum (FCS), but we know that FCS cannot be used if DCs are to be used in clinical treatment. However, if FCS is not used, DC cannot be successfully cultivated. Scientists first thought of replacing 10% of FCS with 10% human autologous plasma, but the results showed that human monocytes were cultured in culture medium containing 10% human autologous plasma, using GM-CSF and IL-4. After 7 days of induction, most of the monocytes remained adherent and the number of semi-suspended iDCs was very small. Later, after continuous exploration, it was found that the addition of 1% human autologous serum to serum-free medium was the best for the induction of iDC from monocytes. A more difficult step is how to induce iDC into mDC in a bovine serum-free culture system. TNF-α and IL-1β are good DC maturation inducers in bovine serum-containing culture systems, while in bovine serum-free culture systems, both are ineffective or weak, ie in a bovine serum-free culture system. TNF-α and IL-1ï¢ do not induce iDC to become mDC, or only a small fraction of iDC can be induced to become mDC.
Later studies showed that the culture supernatant obtained by culturing monocyte-conditioned medium (MCM), which is mononuclear cells on bovine serum coated with immunoglobulin for 24 hours, can be obtained. Full maturation of DC was induced in a bovine serum-free culture system. Therefore, 1% of human autologous plasma + MCM may be the standard method for obtaining mature DCs clinically.
However, the consistency of each prepared MCM is difficult to guarantee, and each batch of MCM must be tested to determine the most suitable amount, which brings resistance and instability to clinical applications. Therefore, people have been looking for a proven combination of mature ingredients that can replace MCM.
In 1997, Jonuleit of Mainz University in Germany made breakthroughs. They found that the combination of cytokines such as TNF-α, IL-1β and IL-6 can completely replace MCM and culture in bovine serum-free. The system is capable of inducing iDC into a fully mature DC.
4. PGE2 and DC:
Jonuleit of the University of Mainz in Germany has proved that the combination of three pro-inflammatory factors such as TNF-α, IL-1β and IL-6 can completely replace MCM, and it can also completely induce maturation of iDC in a bovine serum-free culture system. Another inflammatory mediator, PGE2, further increases DC yield, maturity, migration, and immune activation, meaning that PGE2 can be more mature in addition to TNF-α, IL-1β, and IL-6. Efficient DC, which will improve the therapeutic effect of clinical DC.
The most important reason why PGE2 is often added to the DC maturation induction combination is that it enhances the migration ability of mature DCs, which makes DCs more accessible to lymph nodes, thereby causing immune responses and treating tumors. But we should also pay attention to the following points:
1) PGE2 cannot be added to Step 1 in which DC induces differentiation. If added, differentiation of DC will be inhibited;
2) We know that DCs that induce Th1 responses in the body (known in many literature as DC1) contribute to the treatment of a variety of tumors, such as melanoma and malignant glioma.
Experimental studies have shown that the addition of PGE2 to the mature inducing factor (TNF-α/IL-1β) tends to induce iDC to become mature DC2, but not DC1, which induces a Th2 response. So many people are trying to find the best mature induction combination that can induce DC1, such as TNF-α/IL-1β/IFN-α/IFN-γ/poly-I:C, TNF-α/IL-1β/IFN-γ /PGE2 (low concentration) / R848 (TLR7 / 8 agonist) and IFN-γ / LPS sequential combination, etc., but these new combinations have not been fully clinically validated, so there is no real use for clinical grade DC preparation .
3) In fact, the research results of different families are not the same. For example, the founder of the TNF-α/IL-1β/IL-6/PGE2 combination
--- Jonuleit of the University of Mainz in Germany found that the addition of PGE2 induced mature DCs significantly increased the secretion of IFN-γ during stimulation of T cells, while the secretion of IL-4 and IL-10 was not The effect suggests that the addition of PGE2 induces mature DCs with a propensity to induce a Th1 response.
In conclusion, whether the addition of calf serum to the culture system and the different types of serum-free medium used may lead to differences in the nature of PGE2-induced mature DCs, so it is best to be fully prepared when preparing DC clinically. The previous study then determined the best preparation method for treating a certain tumor.
[ Preparation of DC]
1. Collection of peripheral blood mononuclear cells
1.1 using a blood cell separator to collect the patient's own peripheral blood mononuclear cells 80 - 100ml;
1.2 Lymphocyte separation solution Density gradient centrifugation further purification of mononuclear cells (PBMC).
1.3 Serum-free medium was washed twice to obtain PBMC with a purity of more than 90%, and the number of cells should be 1-3 x 108.
2. (optional step) Preparation of tumor antigen
The tumor antigen for loading DC may be a Tumor-Specific Antigens (TSA) or a Tumor-Associated Antigens (TAA), or may be a tumor whole cell antigen.
DCs loaded with TSA or TAA have good targeting, but the method has the defects that the tumor-specific antigen or antigen peptide species are determined to be small and the immune attack of a single antigen often fails to kill tumor cells. The use of DCs loaded with tumor whole cell antigens overcomes these deficiencies because it is not necessary to know which antigens are TSAs or TAAs of tumor cells, and multiple different tumor antigens in whole antigens can induce DCs to target cells with different antigenic determinants. Toxic T lymphocytes (CTL) are cloned to achieve effective killing of tumor cells.
There are many methods for tumor cell whole antigen-loaded DC, including loading DC with tumor cell lysate, DC with apoptotic tumor cells, DC with necrotic or dead tumor cells, DC with tumor living cells, and DC with tumor cells. Fusion and so on. At present, it is commonly used in clinical practice to load DC with tumor cell lysate, because the method is simple, rapid and effective.
Repeated freezing and thawing is a common method to obtain tumor cell lysate. The specific steps are as follows:
2.1 Surgical removal of tumor specimens, under sterile conditions, remove necrotic tissue and non-tumor tissue adjacent to the tumor;
2.2 Wash 3 times with sterile saline;
2.3 Cut the tumor tissue with a sterile tissue scissors, add RPMI 1640 medium, and grind thoroughly;
2.4 200 mesh sterile mesh filtration after collection of single cell suspension;
2.5 Resuspend the cells in RPMI 1640 medium to 1-2 x 107/ml and place in a 5 ml sterile cryotube;
2.6 The frozen tube was immersed in liquid nitrogen for quick freezing, taken out after 10 min, and then quickly thawed in a 37oC water bath for 10 min. Repeat 3-5 times;
Note: You can also freeze and thaw 3-5 times at -80oC/37oC.
2.7 Add the tumor lysate to the centrifuge tube, centrifuge at 3000 rpm for 10 min;
2.8 Collect the supernatant, filter and sterilize by 0.22ïm filter, and check the protein content and bacteria, fungi and mycoplasma;
2.9 -80oC Save spare.
3. Culture and identification of DC cells
3.1 The PBMC obtained in step 1 was adjusted to a cell concentration of 2 x 106/ml with serum-free medium and placed in a culture flask;
3.2 Incubate for 2 h at 37 ° C in a 5% CO 2 incubator to allow monocytes to adhere;
3.3 Wash off the suspension cells, add serum-free medium containing recombinant human GM-CSF (500-1,000 U/ml) and recombinant human IL-4 (500 U/ml) to adherent cells, culture at 37 ° C, 5% CO 2 Culture in a chamber to induce differentiation of monocytes into DC cells;
3.4 Change the liquid once every 2-3d, and make up the cytokines;
3.5 (optional step) On the 5th day of culture, add the tumor antigen 50 μg/ml obtained in step 2, and carry on antigen loading on DC;
Note: This step is omitted if the antigen load is not applied to the DC.
3.6 On the 6th day of culture, recombinant human TNF-α (10 ng/ml), IL-1β (10 ng/ml), IL-6 (1000 U/ml) and PGE2 (1 μg/ml) were added to induce DC cell maturation;
3.7 On the 7th or 8th day of culture, DC cells should be harvested in an amount of 1×106 or more;
3.8 DC quality inspection:
3.8.1 Proportion of living cells: Trypan blue staining should verify that living cells should be above 90%;
3.8.2 Morphological observation: >90% of cells are semi-suspended, and the cells have multiple dendritic protrusions;
3.8.3 Cell phenotypic analysis (see the figure below): Flow cytometry detects the expression of CD14, HLA-DR, CD40, CD80, CD83 and CD86 on DC cells, and mature DC does not express CD14, but high expression. molecular. CD83 is a specific marker of mature DC and is not expressed or underexpressed on the surface of monocytes and immature DCs.
3.8.4 Sterility test: Take a small amount of culture for bacterial and fungal culture before harvesting cells, and test for mycoplasma and chlamydia, which should be negative;
3.8.5 Endotoxin test: Take a small amount of culture before harvesting the cells, and test the endotoxin content with sputum reagent before returning. Standard: endotoxin <0.5 EU/ml. 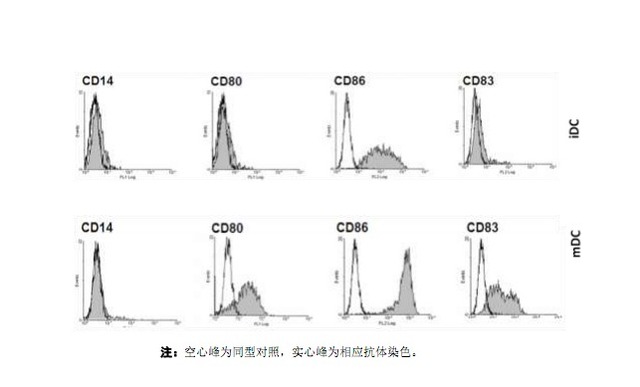
[DC culture reagent recommended]
| manufacturer | product name | Product number | Product specifications | Use concentration |
| PeproTech | Recombinant Human GM-CSF (Animal Free) | AF-300-03 | 5mg/20mg/50mg/100mg/250mg/500mg/1mg | 50-100ng/ml |
| PeproTech | Recombinant Human IL-4 (Animal Free) | AF-200-04 | 5mg/20mg/50mg/100mg/250mg/500mg/1mg | 100ng/ml |
| PeproTech | Recombinant human TNF-a (Animal Free) | AF-300-01A | 10mg/50mg/100mg/250mg/500mg/1mg | 10ng/ml |
| PeproTech | Recombinant Human IL1-b (Animal Free) | AF-200-01B | 2mg/10mg/50mg/100mg/250mg/500mg/1mg | 10ng/ml |
| PeproTech | Recombinant Human IL-6 (Animal Free) | AF-200-06 | 5mg/20mg/50mg/100mg/250mg/500mg/1mg | 100ng/ml |
| PeproTech (BioGems) | PGE2 (prostaglandin E2) | 3632464 | 10mg/50mg | 1mg/ml |
Note: Animal Free means no animal content. The animal-free recombinant cytokine does not have any animal-derived substances in the production process, especially the incorporation of bovine pathogens and proteins, so that the recombinant human protein finally obtained does not contain any animal components. This can avoid contamination of animal pathogens (such as mad cow disease, Creutzfeldt-Jakob disease, etc.) and xenogeneic rejection and allergic reactions caused by foreign proteins. Therefore, it is preferable to use animal-free recombinant cytokines in cell culture in vitro.
[DC identification reagent recommended]
| manufacturer | product name | Product number | Clone number | Fluorescent label | Product specifications |
| PeproTech (BioGems) | Anti-human CD14 fluorescently labeled antibody | 6211 | 61D3 | FITC/PE/APC/PerCP-Cy5.5 | 25tests/100test |
| Anti-human CD40 fluorescently labeled antibody | 2511 | 5C3 | FITC/APC | 25tests/100test | |
| Anti-human CD80 fluorescently labeled antibody | 2911 | 2D10.4 | FITC/PE | 25tests/100test | |
| Anti-human CD83 fluorescently labeled antibody | 5911 | HB15e | FITC/PE | 25tests/100test | |
| Anti-human CD86 fluorescently labeled antibody | 8911 | IT2.2 | PE | 25tests/100test | |
| Anti-human HLA-DR fluorescently labeled antibody | 74111 | LN3 | FITC/PE/APC/PerCP-Cy5.5 | 25tests/100test |
Note: The expression of surface markers for DC identification presents a single peak on the flow chart and in most cases cannot be completely distinguished from the negative peak. In order to avoid the inaccuracy of the results caused by poor compensation adjustment in multi-color analysis, it is recommended to use single label, up to double label for DC surface marker analysis, and must use the same type of control instead of blank cell control to exclude the background. dyeing.
ã€references】
[1] Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973; 137 (5): 1142–62.
[2] Bender A, Sapp M, et. al. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996; 196(2): 121-35.
[3] Romani N, Reider D, et. al. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996; 196(2): 137-51.
[4] Jonuleit H, Kühn U, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997; 27(12):3135-42.
[5] Datta J, Terhune JH, et al. Optimizing dendritic cell-based approaches for cancer immunotherapy. Yale J Biol Med. 2014; 87(4): 491-518.
[6] de Jong EC, Vieira PL, et al. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse th cell-polarizing signals. J Immunol. 2002 Feb 15;168(4): 1704-9.
[7] Zhang Zhiwei, Song Xin. Standardized study of clinical preparation of DC-CIK cells. Chinese Journal of Oncology, 2011; 20(2): 85-88.
Milk Thistle extract powder silymarin is a native to central and western Europe, especially close to the Mediterranean, that was introduced and naturalized in California and other parts of the United States of America. It is found mainly on dry rocky or stony soils in wastelands, especially by buildings, hedge banks, fields and by roadsides up to an altitude of around 600 metres or 2000 feet. The Milk thistle is an annual or biennial, its height is from 30 to 150 cm (1 foot to 4 feet) tall, it has a seldom branched erect stem that is prominently grooved. It has large leaves that are oblong, smooth and shiny, marked with white veins.
Silymarin is kind of a flavonoid lignans compounds extracted from the seed coats of the medicinal compositae Silybum marianum, yellow or brown powder, bitter in taste. Ethyl Acetate, Ethanol and Methanol, Hardly soluble in chloroform, insoluble in water. The main ingredients are : Silybin, Isosilybin, Silydianin, Silychristin, etc
Milk Thistle Extract,Milk Thistle Extract Powder,Milk Thistle Extract Silymarin,Milk Thistle Extract Powder Silymarin
XI'AN PLANT BIO-ENGINEERING CO.,LTD , https://www.plantbic.com