Significantly increased platelet levels, new clinical trials of blood diseases announced phase 3 clinical results
May 03, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, Rigel Pharmaceuticals announced that the positive results obtained by the company's fostamatinib in the Phase III trial of the treatment of chronic immune thrombocytopenia (ITP) were published in the scientific journal American Journal of Hematology. Fostamatinib is a small molecule inhibitor of spleen tyrosine kinase (Syk) developed by Rigel. Recently, it has been approved by the US FDA for the treatment of patients with chronic ITP who have responded poorly to previous therapies.

ITP is a chronic disease caused by the immune system attacking and destroying its own platelets. Due to the important role of platelets in blood clotting and wound healing, common symptoms of ITP patients are excessive congestion, bleeding and fatigue. Patients with ITP may have a significantly increased risk of severe bleeding from hospitalization or death. Currently, existing treatments for ITP include steroids, drugs that promote platelet hyperplasia, and spleen resection. However, many patients have a poor response to existing therapies, so developing more treatments for ITP remains an unmet medical need.
Fostamatinib is an oral small molecule Syk inhibitor. When autoantibodies targeting platelets bind to platelets, their Fc ends destroy platelets by activating Fc receptors that trigger phagocytosis. Syk plays an important role in cell proliferation, differentiation, immune regulation, and cytoskeletal reorganization during phagocytosis. Moreover, Syk is involved in the signaling pathway of B cell receptors and may be involved in the regulation of autoantibody production. In the mouse ITP model, inhibition of Syk with fostamatinib significantly reduced platelet damage.
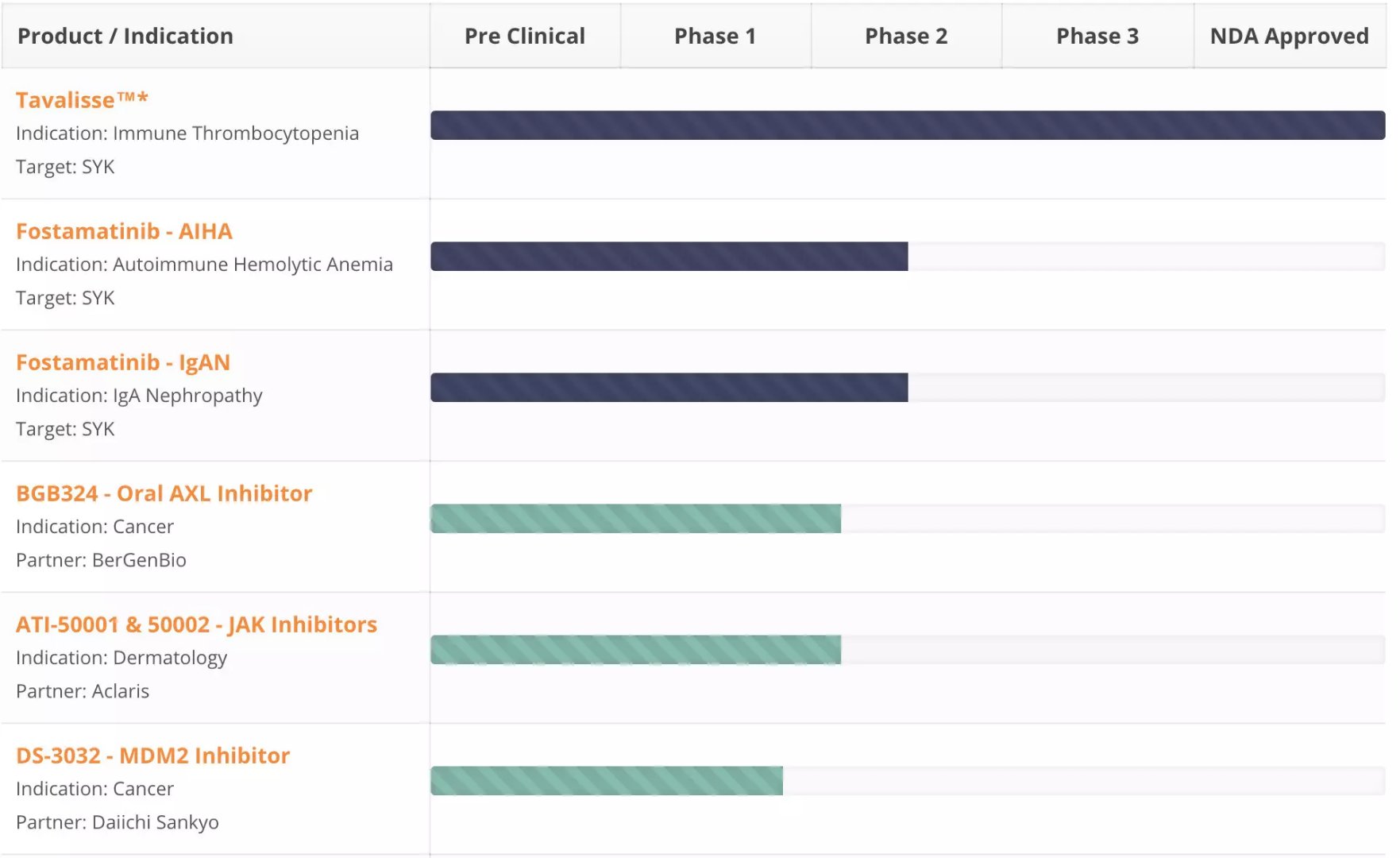
â–² Rigel Pharmaceuticals part of the research and development pipeline (Source: Rigel Pharmaceuticals official website)
In two parallel, multicenter, placebo-controlled, randomized, double-blind, phase 3 trials with FIT1 and FIT2, a total of 150 chronic ITP patients were randomized to a fostamatinib treatment group and a control group at a 2:1 ratio. These patients have an average ITP duration of more than 8 years and have received an average of 3 different treatments. They were given twice daily with 100 mg of fostamatinib or placebo. The test period is 24 weeks.
The results of the trial showed that 18% of patients receiving fostamatinib had a platelet level of more than 50,000/μl after 14 weeks of treatment, and only 2% of patients in the control group (P=0.0003). Forty-three percent of patients treated with fostamatinib had platelet levels of more than 50,000/μl within 12 weeks of treatment, compared with only 14% of patients in the control group (P=0.0006). The patient's mean baseline platelet level was 16,000 per microliter before treatment. Therefore, fostamatinib can significantly increase platelet levels in certain ITP patients.
The most common side effects of fostamatinib therapy observed in clinical trials are increased diarrhea, hypertension, nausea, dizziness, and ALT, with most side effects being mild or moderate. These side effects may disappear automatically or may be controlled after receiving medication.

â–² Dr. Anne-Marie Duliege (Source: LinkedIn)
"These data demonstrate that fostamatinib may increase platelet levels quickly, strongly, and permanently. We are very pleased to be able to deliver this option to ITP patients who need other therapies in the near future," Dr. Anne-Marie Duliege, Chief Medical Officer, Rigel "Fostamatinib is the first FDA-approved therapy to target the Syk signaling pathway, and Syk is the primary signaling pathway that mediates the destruction of platelets in patients with ITP."
Reference materials:
[1] TAVALISSETM (fostamatinib disodium hexahydrate) Phase 3 Data Published in the American Journal of Hematology Describes Pivotal Data and Overall Response Rate Versus Placebo
[2] Fostamatinib for the Treatment of Adult Persistent and Chronic Immune Thrombocytopenia: Results of Two Phase 3, Randomized, Placeboâ€Controlled Trials
Natural pigments are food pigments obtained from natural resources. Pigments extracted mainly from animal and plant tissues and microorganisms (cultures), in which vegetative colorants predominate. Natural pigments not only have the function of coloring food, but also have physiological activity. At present, there are 48 kinds of edible natural pigments approved to be used in China, commonly used chili red, beet red, monascus red, cochineal red, gaolianghong, sodium copper chlorophyllin, turmeric, gardenia yellow, carotene, algal blue pigment, cocoa pigment, caramel pigment and so on.
Roselle Calyx Extract,Phycocyanin,Lutein,Butterfly Pea Flower Powder,Beta Carotene Powder,Sodium Copper Chlorophyllin
Xi'an Gawen Biotechnology Co., Ltd , https://www.amulyn-bio.com