Significantly improve sleep efficiency, clinical results of insomnia new drugs are positive
February 01, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Sage Therapeutics today announced the positive results of the SAGE-217 Phase 1/2 clinical trial, which showed that the therapy significantly improved sleep efficiency (SE) in patients with insomnia, prolonged total sleep time and sleep maintenance time. Based on these positive results, Sage is expected to begin developing SAGE-217 for sleep disorders in 2018.

Insomnia is clinically defined as an unsatisfactory quality of sleep and a serious negative impact on daytime work. Insufficient sleep can seriously affect the physical function and physical and mental health during the day, leading to fatigue, daytime sleepiness, inattention, low mood, or inability to perform tasks. Insomnia is the most common sleep disorder worldwide. According to GlobalData's 2015 study, by 2020, there will be approximately 13 million insomnia patients in the United States who need medication.
SAGE-217 is the next generation of forward allosteric modulators optimized for the selectivity of synaptic and extrasynaptic GABA receptors and the pharmacokinetic profile of daily oral administration. The GABA system is the main inhibitory signaling pathway in the brain and central nervous system, and is important for regulating central nervous system function. SAGE-217 is currently being developed for major depression and certain other mood and movement disorders.
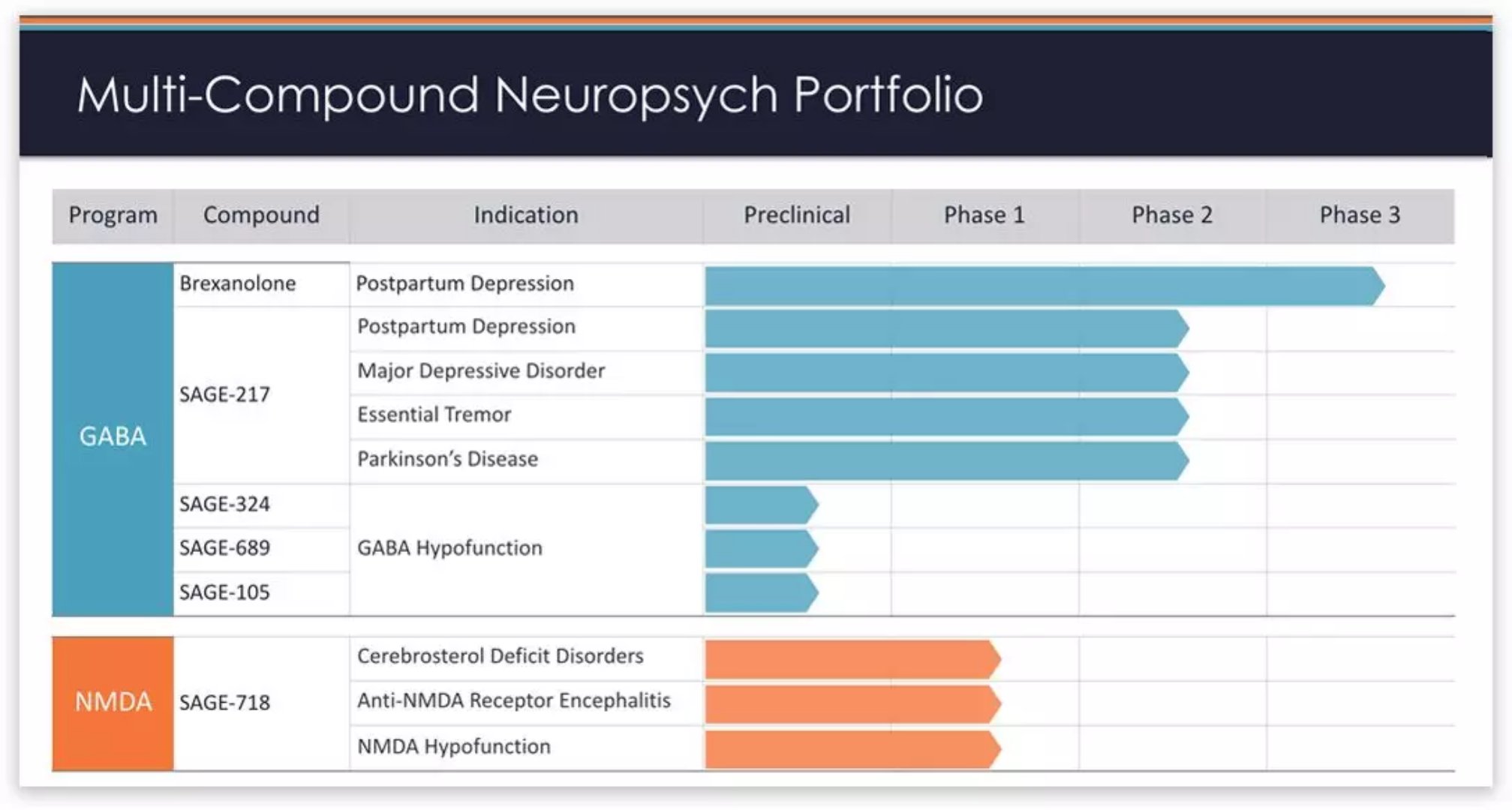
â–²Sage Therapeutics R&D pipeline (Sage Therapeutics official website)
In this double-blind, placebo-controlled, crossover study, healthy volunteers (n = 45) were randomized to receive 30 mg or 45 mg doses of SAGE-217 or placebo. The first treatment period was from the first day of study to the second day, using a 5-hour phase advance model of insomnia (lights off 5 hours before the participants were used to bedtime and began polysomnography). Study medication (SAGE-217 30 mg, 45 mg or placebo) was given 30 minutes before the lights were turned off and participants were asked to stay in bed for 8 hours. A similar procedure was followed for the second treatment period (study days 8 to 9) and the third treatment period (days 15 to 16 of the study).
This study reached the primary endpoint and significantly improved sleep efficiency (SE). Single-dose SAGE-217 at 30 and 45 mg improved sleep efficiency to 84.64% (p < 0.0001) and 87.55% (p < 0.0001), respectively, as measured by polysomnography, compared with 72.92% of the placebo group. The secondary end point sleep awakening (WASO) was reduced from the placebo group at 113.0 minutes to 55.0 minutes (p < 0.0001) (30 mg) and 42.5 minutes (p < 0.0001) (45 mg). In addition, SAGE-217 also extended total sleep time (another secondary endpoint) to 406.25 minutes (p < 0.0001) (30 mg) and 420.25 minutes (p < 0.0001) (45 mg) compared to placebo The median sleep time of the subjects was 350.00 minutes. SAGE-217 was well tolerated in this study with no serious adverse events. Comprehensive data from the study, including additional secondary endpoint data, will be presented at future scientific conferences.

â–² Dr. Jeff Jonas, CEO of Sage Therapeutics (Sage Therapeutics official website)
Dr. Jeff Jonas, CEO of Sage Therapeutics, said: “Whether as a primary disease or related to other diseases, sleep disorders have a profound impact on the quality of life of many people. For the treatment of sleep dysfunction, we need to improve the patient experience, we The opportunity to develop potential solutions was determined using the GABA mechanism of action. SAGE-217 has unique potential for a variety of psychiatric and neurological disorders in relation to related symptoms, including mood, sleep and motor dysfunction."
Dr. Jim Doherty, Principal Investigator of Sage Therapeutics, said: "The key to Sage's experimental medical capabilities is the deep insight between the compound and the indication, and the higher likelihood that the drug in the pipeline will be successful. We have multiple clinical trials. The assessment of SAGE-217 suggests that the drug's mechanism of action may rebalance the underlying brain circuit to support the development of SAGE-217 in a variety of diseases. These findings suggest that SAGE-217 helps maintain sleep, we Both tests were satisfactory for both doses."
We expect this drug to allow more people with sleep disorders to fall asleep.
Reference materials:
[1] On a Roll: Sage Shows Off Early-Stage Insomnia Data
[2] Sage Therapeutics Announces Positive Results from Placebo-Controlled Trial in a Model of Insomnia Demonstrating Activity on Sleep Parameters and Supporting Development of SAGE-217 as Potential Treatment for Sleep Disorders
The lock channel is one of the most important thing for greenhouse, it can lock the film ,shading net ,insect net .
It is hot galvanized or aluminum material.
We have different thickness.
Welcome to contact us
Lock Channel,Greenhouse Lock Channel,Channel Lock,greenhouse profile
JIANGSU SKYPLAN GREENHOUSE TECHNOLOGY CO.,LTD , https://www.engreenhouse.com