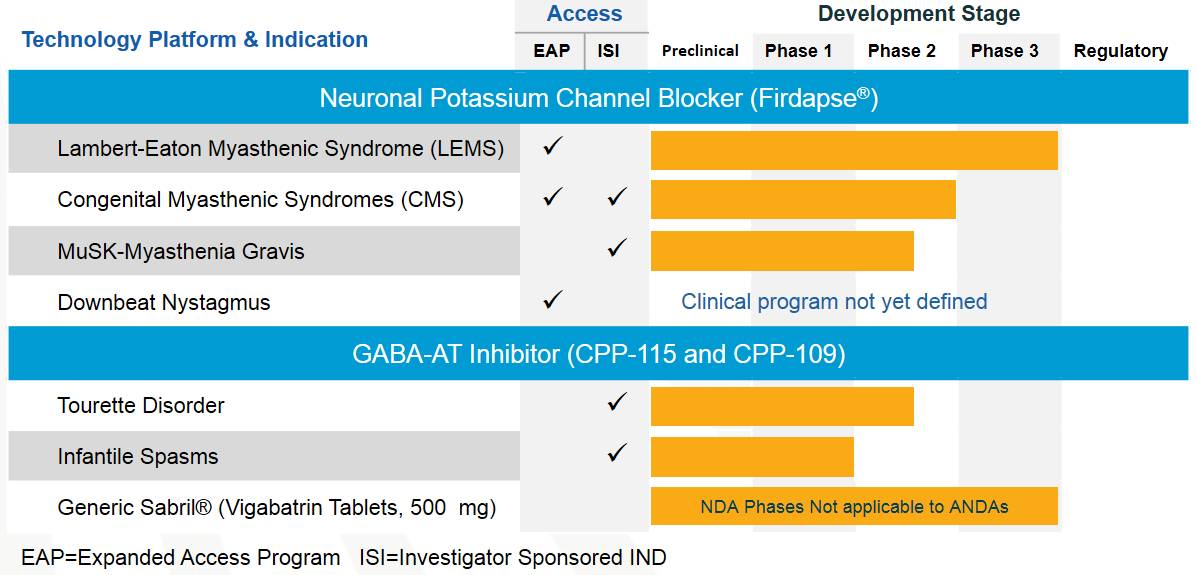
Myasthenia gravis new drug Firdapse gets positive phase 3 clinical results
November 28, 2017 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Catalyst Pharmaceuticals today announced the top-line results of Firdapse (amifampridine) Phase 2 clinical trial LMS-003, which used 10 mg amifampridine phosphate tablets to treat Lambert-Eaton myasthenic syndrome (LEMS).

LEMS is a rare autoimmune disease, the most common feature being muscle weakness in the limbs. The cause of this disease is due to the formation of antibodies in the nerves and the muscle connections with which they interact, impeding voltage-gated potassium channels. In approximately 50% of cases, LEMS is associated with a potential malignancy, most commonly small cell lung cancer. In some patients, LEMS is the first symptom of this malignancy. LEMS usually affects the ends, especially the legs. Since this disease most affects the limbs close to the torso, it can hinder daily life such as climbing stairs or sitting up.
Firdapse is 3,4-diaminopyridine phosphate (3,4-DAP), which is approved by the FDA for breakthrough therapy identification and orphan drug status. And approved for treatment of LEMS in Europe.
â–²Firdapse is the main product of Catalyst (Source: Catalyst official website)
The LMS-003 clinical trial was a randomized, double-blind "withdraw trial" in which LEMS patients who received Firdapse in the Catalyst Extension Program (EAP) were screened after completing Firdapse or placebo (randomly assigned, 1:1) , was invited to participate in the LMS-003 test. A total of 26 patients completed randomized treatment. The trial was designed to achieve clinical significance when the symptoms of the placebo group worsened. The two common primary endpoints of the trial were the QMG score and the SGI score. The QMG score is a semi-quantitative assessment by a physician and consists of 13 assessments (0 to 3 for each score), including arm strength, leg strength, facial and neck muscle performance, swallowing, speaking, grip, forced breathing. And gaze disorder tests, the total of these assessments is the QMG score. The SGI score is the patient's satisfaction rating for their LEMS symptoms, ranging from 1 (bad) to 7 (satisfactory).
Both primary endpoint scores were statistically significant (QMG p=0.0004, SGI p=0.0003). More importantly, a clinically significant difference of 6.4 points was observed at the QMG endpoint between the Firdapse group and the placebo group. Firdapse is well tolerated and shows similar safety to earlier studies. All p values ​​reported were based on the overall intention to treat (ITT) patient population participating in the trial in this trial. The secondary end point of the prospective physician's clinical overall improvement impression (CGI-I) was statistically significant (p=0.0020). In addition, the exploratory endpoint results included: (1) the three-way walking trial (3TUG) endpoint reached statistical significance (p=0.0112), and (2) the QMG-limb limb endpoint reached statistical significance (p=0.0010) (3) The annoying symptom (MBS) endpoint did not reach statistical significance, but showed a positive trend (p=0.0572).
"The data from the LMS-003 trial continues to demonstrate that amifampridine phosphate has a significant effect on the symptoms of patients with LEMS." Dr. Perry Shieh, associate professor of neurology at David Geffen School of Medicine at the University of California, Los Angeles (UCLA), said the principal investigator of the clinical trial said: “This finding is especially relevant given that FDA-approved therapies may change the lives of LEMS patients.â€

â–² Mr. Patrick J. McEnany, President and CEO of Catalyst (Source: Catalyst's official website)
“We are very pleased with the top-line efficacy and safety results of the second Phase 3 clinical trial, which enhances Firdapse's important therapeutic potential for LEMS patients. We look forward to exposing more data in future publications and medical conferences, We are expected to submit a new drug application to the FDA in the first quarter of 2018," said Patrick J. McEnany, President and CEO of Catalyst Pharmaceuticals. "Catalyst is committed to developing new treatments to improve the function and life of patients with rare neurodegenerative diseases. â€
Dr. Gary Ingenito, Chief Medical Officer of Catalyst Pharmaceuticals, said: "Catalyst has once again demonstrated that Firdapse can produce positive clinically significant results in the treatment of LEMS. We are grateful to all those who participated in the LMS-003 trial in order to get LEMS patients with FDA-approved drugs. â€
We expect this new drug to pass the FDA review and meet patients early to improve the lives of patients with this rare disease.
Reference materials:
[1] Catalyst Pharma's Firdapse Scores Win in Second Phase III LEMS Study
[2] Catalyst Pharma's official website
Original title: It is expected to be approved next year, and the new drug of muscle weakness will receive positive phase 3 clinical results.
Dietary supplement
Fufeng Sinuote Biotechnology Co.,Ltd. , https://www.sntbiology.com