

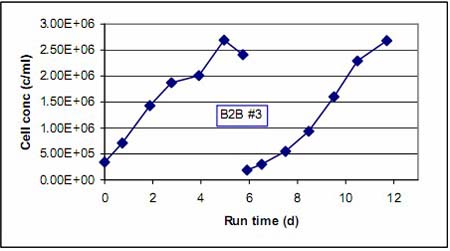

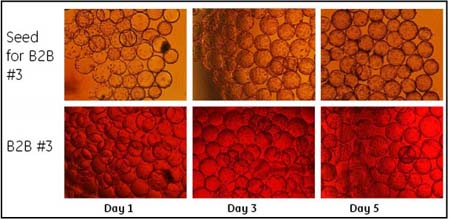



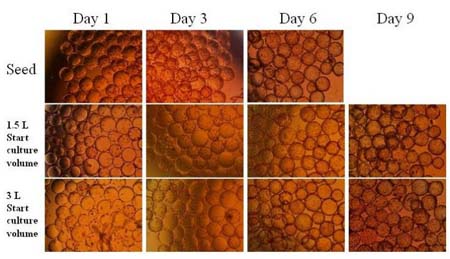
We are also able to offer solutions for the meat industry, dairy, for the fish and seafood industry and for other sectors such as catering, gastronomy, bakery and bolleria or sports and diet nutrition.
If
you want to know more about the products in Prepared For Meat Treatment, please click the product details to
view parameters, models, pictures, prices and other information about Prepared for meat treatment.
Whatever you are a group or individual, we will do our best
to provide you with accurate and comprehensive message about Prepared For Meat Treatment!
TGase prepared for meat treatment, TG enzyme for sausage, Transglutaminase enzyme for sausage
Guangdong Kelong Biotechnology Co., Ltd. , https://www.kelongfood.com