Tumor immunotherapy in the study of drug targets overview
December 11, 2017 Source: Author drugs crossing: clear water and pure heart
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];
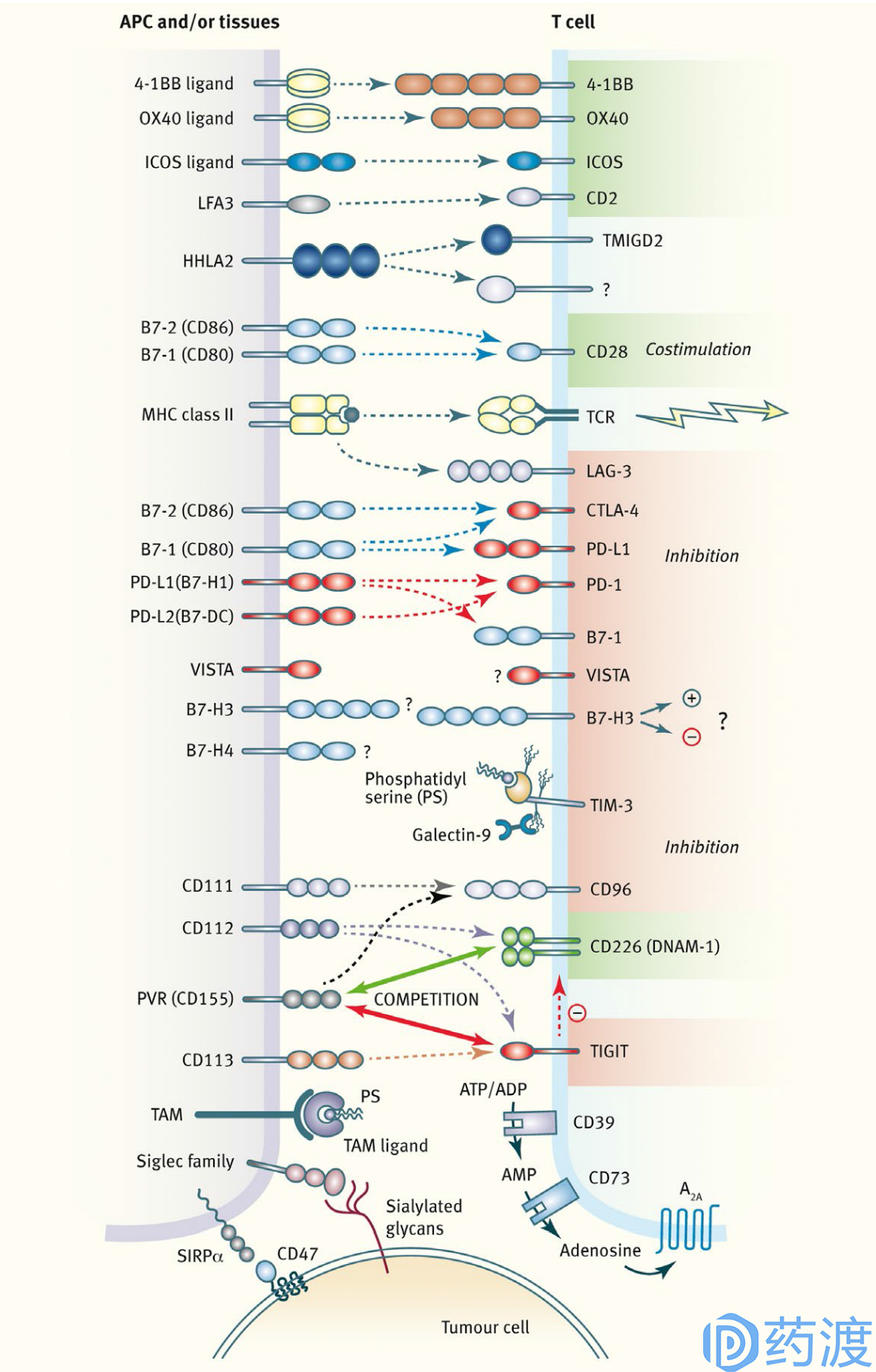
Overview of innate and adaptive checkpoint pathways
Immunotherapy refers to the treatment of cancer through the immune system and is also a form of biological therapy. Identifying and killing abnormal cells is a natural property of the immune system, but cancer cells often have the ability to evade the immune system. In the past few years, the rapid development of cancer immunology has produced several new ways to treat cancer by enhancing the activity of certain components of the immune system or relieving the inhibition of the immune system by cancer cells. Generalized immunotherapies include immunological checkpoint inhibitors, immune cell therapy, oncolytic viruses, therapeutic antibodies, cancer vaccines, and immune system modulators. Tumor immunotherapy has recently received much attention. In addition to surgery, chemotherapy and radiotherapy, it has become an important means of cancer treatment. Ipilimumab and nivolumab / pembrolizumab are the earliest listed immunological checkpoint inhibitors, targeting CTLA-4 and PD-1, respectively. They have gradually changed from second-line to first-line treatment in the treatment of non-small cell lung cancer and melanoma. Immunotherapy drugs are not without weaknesses. The trial of drugs may induce the up-regulation of additional immune checkpoint activity in tumor cells, highlighting the importance of developing new anti-tumor immune activators. Emerging drugs not only target acquired immune lymphocytes by blocking immunosuppressive checkpoints or activators as immunostimulatory signaling pathways, but also target macrophages and natural killer cells in innate immune processes, It is widely found in many types of solid tumors and hematological cancers. This article will provide an overview of emerging tumor immunotherapy targets for clinical or preclinical studies.
Table 1. Summary of past and current clinical trials in cancer for immune checkpoint agents
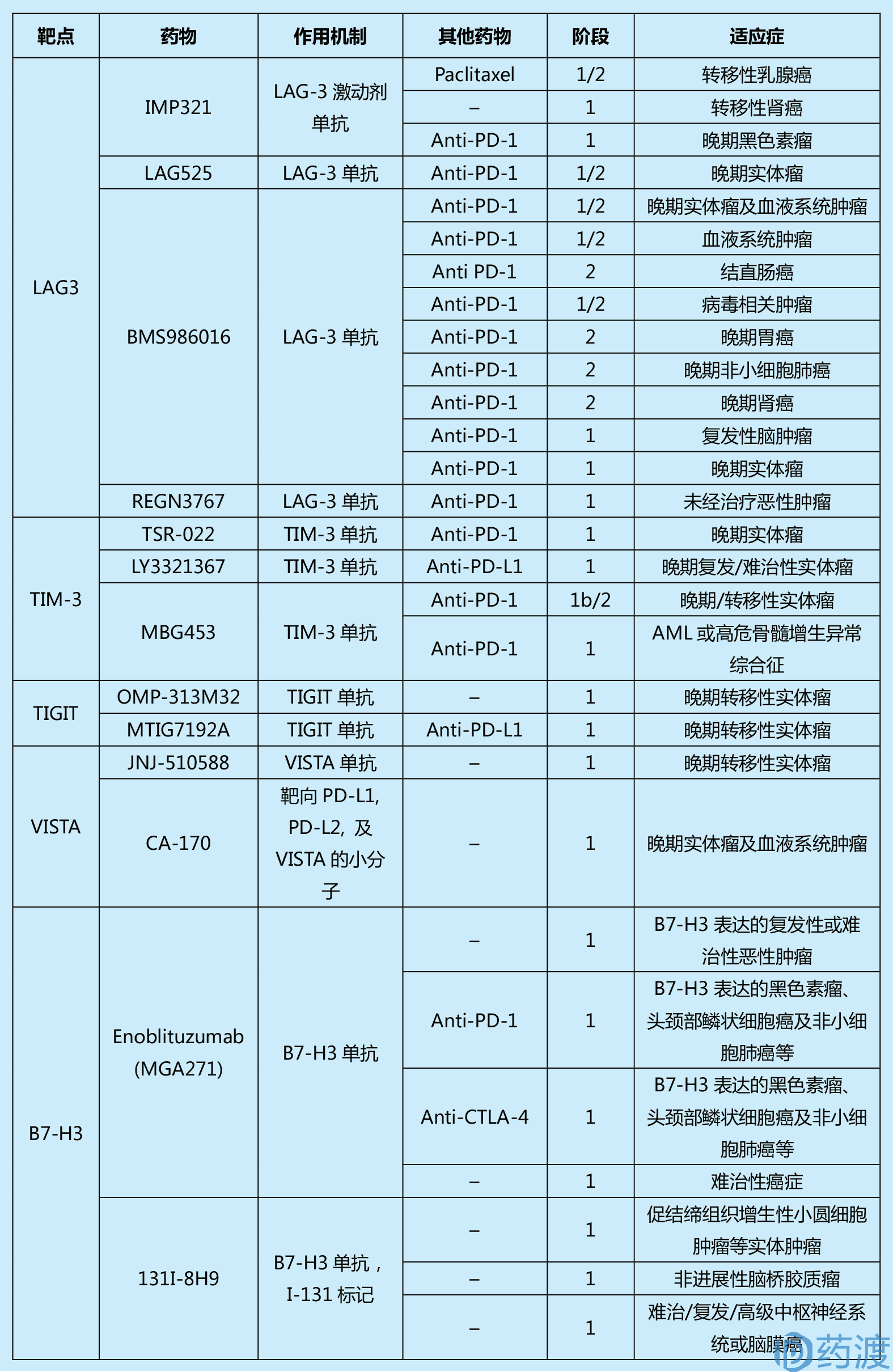
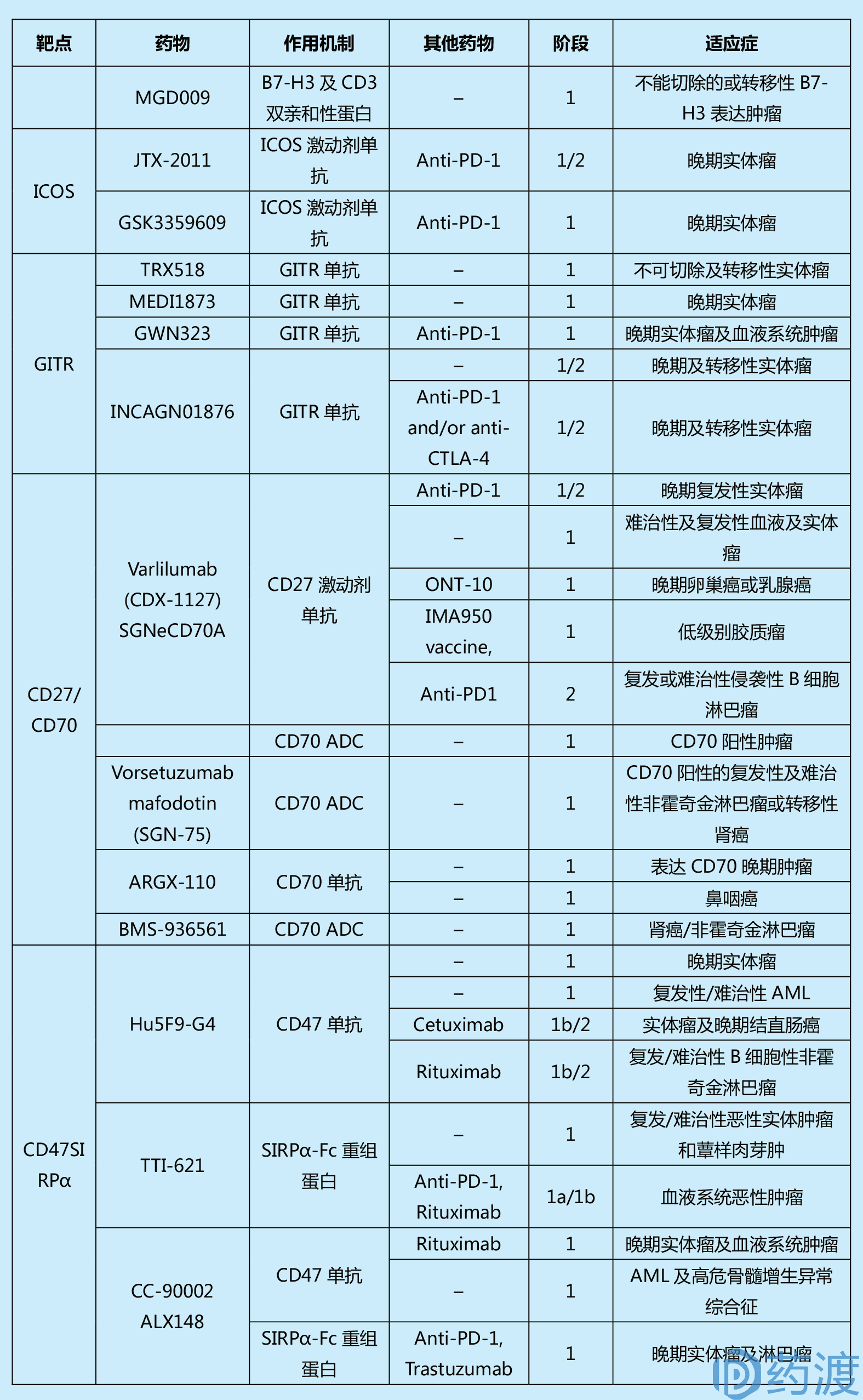
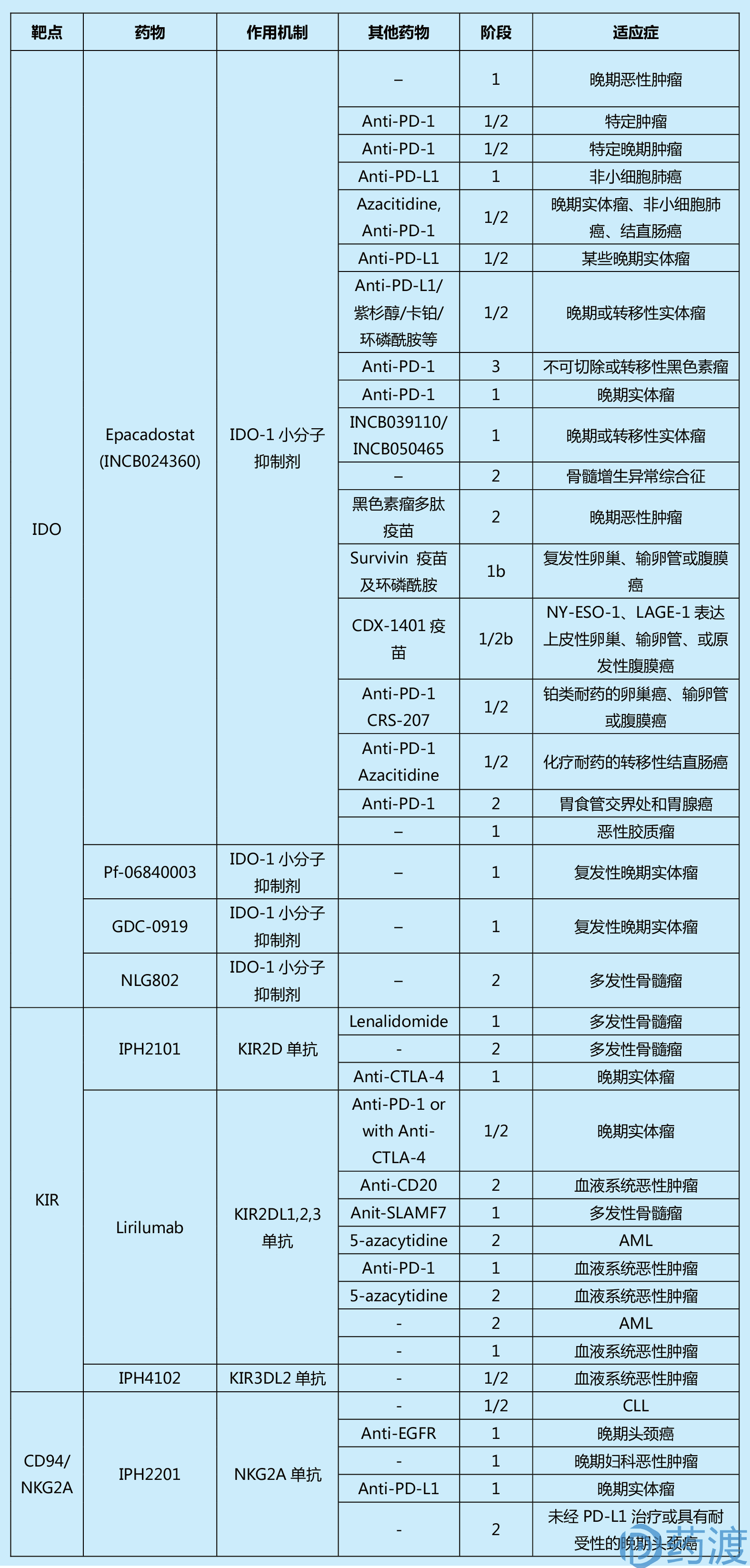
(This table is from the literature 1, limited by space, readers who are interested in more information can refer to the original)
Acquired immune- suppressive lymphocyte receptor
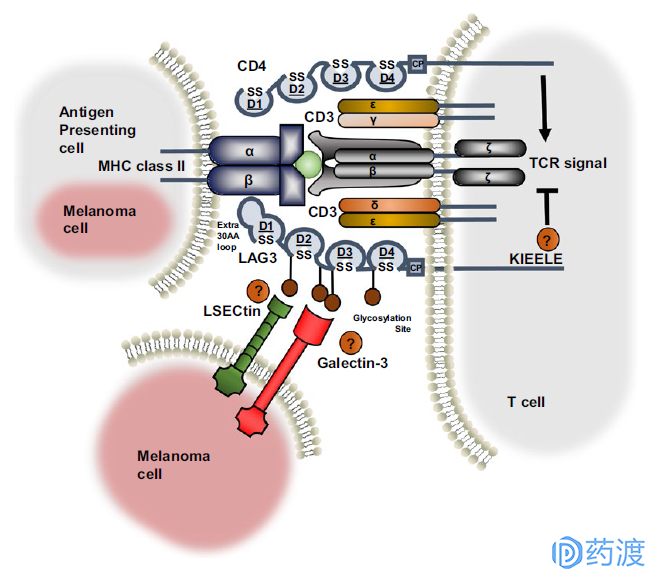
Ligand interaction and structural similarities between LAG3 and CD4
LAG-3
LAG-3 (lymphocyte activation gene 3, LAG-3, CD223) and CD4 are homologous proteins, but bind to major histocompatibility complex class II (MHCII) with higher affinity, mainly It is expressed in activated T lymphocytes, B lymphocytes, natural killer cells (NK) and plasma cytoid dendritic cells (pDCs), and negatively regulates T cell function. Studies have shown that LAG-3 selectively upregulates CD4 on the surface of Treg, and thus LAG-3 antibodies reduce Treg activity in vivo. Inhibition or knockdown of LAG-3 will abolish the inhibitory function of Treg on T cells. In addition, in the absence of CD4+ T cells, LAG-3 antibodies are able to increase the function of CD8+ T cells. When T cells are disabled or depleted, they will express a variety of immune checkpoint molecules. In chronic infection models and autoantigen recognition models, LAG-3 and PD-1 usually have co-expression. Coordinated inhibition of LAG-3 and PD-1 enhances the immune response, so current clinical trials of LAG-3 antibodies are used alone or in combination with PD-1. At present, there are mainly the following companies to carry out clinical trials of LAG-3: BMS986016 of BMS, REGN3767 of Regeneron and Sanofi, LAG525 of Novartis and IMP321 of Immunpet.
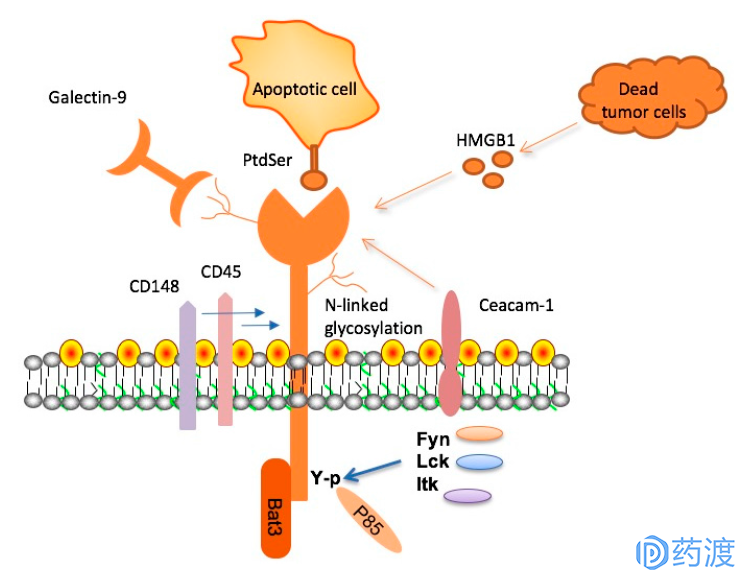
TIM-3, its ligands and signaling adaptor proteins
TIM-3
TIM-3 is a receptor protein of the TIM family and is expressed on the surface of T cells, Treg cells, and innate immune cells (dendritic cells, natural killer cells, and monocytes). TIM-3 has a variety of ligands such as phosphatidylserine, galectin-9, HMGB1 and CEACAM-1. Unlike other immunological checkpoint molecules, TIM-3 is not upregulated after all T cell activation, and is up-regulated only in CD4+ helper T cell 1 (Th1) and CD8+ cytotoxic T cells, and participates in synergistic inhibition. Upon activation by its ligand galectin-9, TIM-3 inhibits the activity of effector T cells and causes peripheral tolerance. TIM-3 plays a key role in the loss of T cells in tumors. TIM-3 is highly expressed in T cells of animals resistant to anti-PD-1 treatment. In an independent experiment, anti-TIM-3 antibodies inhibit the development of anti-PD-1 treatment resistance when used in combination with anti-PD-1 drugs. The TIM-3 antibodies currently in clinical trials include Tesaro's TSR-022, Novartis MBG-453, and Lilly's LY3321367.
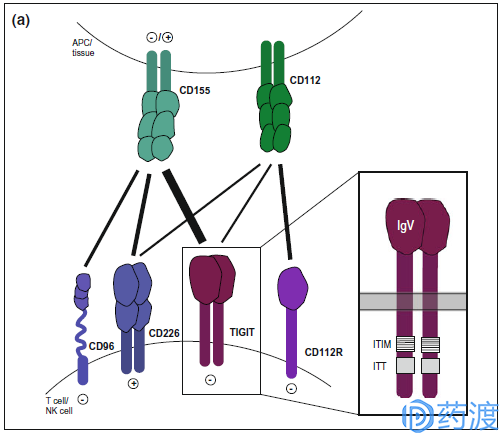
TIGIT pathway
TIGIT
TIGIT (also known as Vsig9, Vstm3, WUCAM) is an inhibitory receptor shared by T cells and NK cells containing Ig and ITIM domains. It is a type I transmembrane protein, including the extracellular domain of IgV and immunoglobulin tyrosine. Tail-like phosphorylation fragment. TIGIT and CD226 (DNAM-1) compete for binding to ligands CD155 (PVR, NECL5) and CD113 (PVRL3, nectin-3). In vitro blocking of TIGIT enhances the activation and degranulation levels of NK and T cells, and also increases the secretion of cytokines such as IFN-γ; TIGIT expression is significantly up-regulated on NK and T cells in different mouse tumor models. The TIGIT antibodies currently in clinical studies are mainly Genentech's MTIG7192A and Genentech's MTIG7192A / RG6058, either alone or in combination with the PD-L1 antibody Atezolizumab for the treatment of advanced or metastatic tumors.
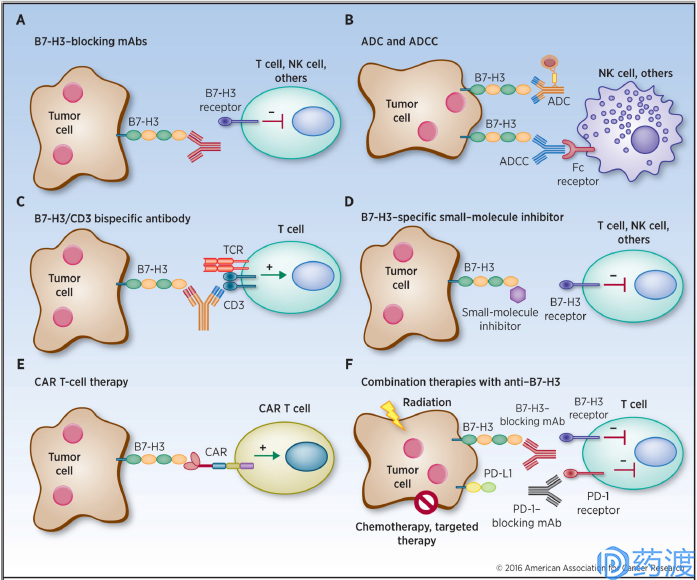
Human cancer immunotherapy strategies targeting B7-H3
B7-H3
Also known as CD276, which is a type I transmembrane protein, the extracellular domain contains two pairs of identical immunoglobulin variable and constant regions, the intracellular region is short, and there is no clear signal motif. The expression of mRNA is more extensive, but the protein expression is relatively limited to non-immune cells such as resting fibroblasts, endothelial cells, osteoblasts, amniotic fluid stem cells, and the surface of induced antigen-presenting cells and NK cells. Many studies have revealed that B7-H3 is overexpressed in a variety of tumors, including melanoma, leukemia, breast cancer, prostate cancer, colorectal cancer and other tumors. The expression level is closely related to poor prognosis and clinical outcomes. Participated in the immune escape of tumors. Although its molecular mechanism is still unclear, it is a promising target for tumor immunotherapy as a possible immune checkpoint molecule.
The anti-tumor effect of MacroGenics' Enoblituzumab (MGA271), which works through antibody-dependent cell-mediated cytotoxicity (ADCC), has entered Phase I clinical trials and has shown encouraging initial results. Antibody-drug conjugates (ADCs) act as biological missiles, and radiolabeled B7-H3 monoclonal antibody 8H9 is successfully used for clinical treatment of metastatic neuroblastoma, while radiolabeled humanized 8H9 antibody is positive Used for clinical trials of peritoneal cancer, glioma, and central nervous tumors. The development of bispecific antibodies, chimeric antibody receptor T cells (CAR-T), and small molecule inhibitors enriches the strategy of tumor immunotherapy, and the combined application promotes synergistic effects, and further studies on its receptors and mechanisms are more effective in design. The foundation of the therapeutic drug.
VISTA
VISTA (V-Domain Immunoglobulin-Containing Suppressor of T Cell Activation) belongs to the immunoglobulin family, and the extracellular domain is homologous to PD-L1. Human VISTA is mainly expressed in CD4+ cells, CD8+ T cells, CD11b+ subpopulations of monocytes, lymphocytes, bone marrow cells, dendritic cell subsets, and neutrophils. The cell surface receptors of VISTA are currently unclear. VISTA has an inhibitory effect on antigen presenting cells and T cells. The current VISTA antibodies in the clinical phase are Johnson JNJ-61610588 and Curis's small molecule drug CA-170.
Acquired immune- lymphocyte costimulatory molecule receptor
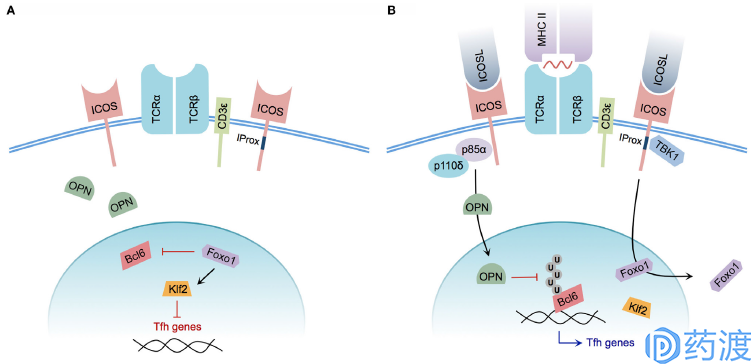
Model of ICOS-mediated Tfh cell differentiation
ICOS and ICOS-L
ICOS is a newly discovered co-stimulatory molecule that induces expression on activated T cells. ICOS is homologous to CD28 and CTLA-4 and is considered to be the third member of the CD28 family. The co-stimulatory signal provided by ICOS can promote T cell proliferation, regulate T cell differentiation, maintain the effect and function of activated T cells (including memory T cells), participate in T/B synergy, and affect Ig type conversion. It is currently believed that CD28 plays an important role in the initiation of the immune response and ICOS plays a more important role in the late phase of the response and in the maintenance effect phase. ICOS ligands ICOSL (also known as B7-H2, GL50, referred to as B7h, B7RP-1 in mice) and B7-1/B7-2 have high homology and are classified as B7 family 5-7. It is mainly expressed on B cells and is also expressed on DC and Mφ. However, ICOSL is not only expressed on hematopoietic cells, but also expressed on other tissue cells when stimulated by inflammation, which is different from B7-1/B7-2. There are currently two types of drug candidates, JTX-2011 and GSK3359609, which are being combined with different PD-1 monoclonal antibodies to conduct a clinical phase I study.
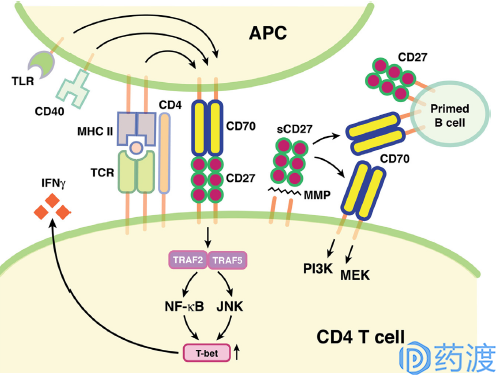
CD27–CD70 pathway in immune regulation
CD27 and CD70
The mechanism of action of the TNF receptor superfamily on immune upregulation is different from the B7/CD28 immunostimulatory interaction. Among the more well-known members is CD27, which is specifically expressed in lymphocytes. When activated, CD27 is abundantly expressed on the cell surface. The CD27 signal is limited by the level of ligand CD70 expression, which is limited by antigen receptor activation by T cells, B cells, and dendritic cells. CD27/CD70 signaling promotes clonal expansion and survival of T cells, promotes effector and memory T cell differentiation, and enhances the activation and function of B cells and NK cells.
In a transgenic mouse model, sustained expression of CD70 activates the CD27/CD70 signaling pathway, upregulates effector T cells and prevents tumor progression. In a pre-clinical mouse model of normal immune function, CD27 agonist treatment also prevents tumor formation and progression. Varlilumab is a fully human-derived monoclonal antibody targeting CD27 and is currently in clinical phase II study for the treatment of malignant melanoma and solid tumors. Clinical Phase I/II studies for the treatment of renal cell carcinoma and treatment of hematological malignancies and prostate cancer Phase I clinical trial. The drug has been clinically tested in the treatment of breast and ovarian cancer, but the study has now been discontinued. For the treatment of CD70, three types of antibody-conjugated drugs and one monoclonal antibody are currently in clinical research.
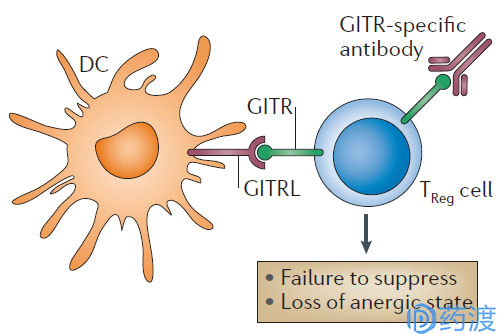
Initial hypothesis concerning the effects of GITR signalling on TReg cells
GITR
GITR refers to a glucocorticoid-induced tumor necrosis factor receptor (GITR) protein that is a new member of the TNFR superfamily. It was originally cloned from Nocentini et al. in hybridoma cell lines in 1997. In 1999, human GITRs similar to murine GITR and their ligands (GITR ligand, GITRL) were cloned by two groups of scientists. So far, people have studied more about the role of GITR signaling in the acquired immune system. The combination of GITR and GITRL can always activate Treg cells and stimulate effector T cells; thus, for tumor immunotherapy, activation of GITR is one of the more promising strategies.
A number of preclinical studies in a solid tumor mouse model have reported the activity of the mouse GITR agonist DTA-1 in vivo. Combinations of GITR agonists with other immunomodulators increase overall anti-tumor effects. One of the most interesting findings is that GITR agonists inhibit tumor growth and increase survival effects not only in immunogenic tumors (colon, bladder, lung model, melanoma), but also in low-immunogenic tumors (breast cancer) In a mouse model of melanoma, ovarian cancer, etc., so that GITR agonists have unique advantages over other immunological checkpoint inhibitors. Immunohistochemistry and flow cytometry experiments showed that GITR is expressed in a variety of human solid tumors. In patients with breast cancer and endometrial cancer, GIRT is expressed more frequently in Treg cells than in peripheral blood. At least four GITR agonist drugs have entered the early stage of clinical research, and the drugs alone have been combined with other immune checkpoint inhibitors.
Innate immunity - macrophage checkpoint
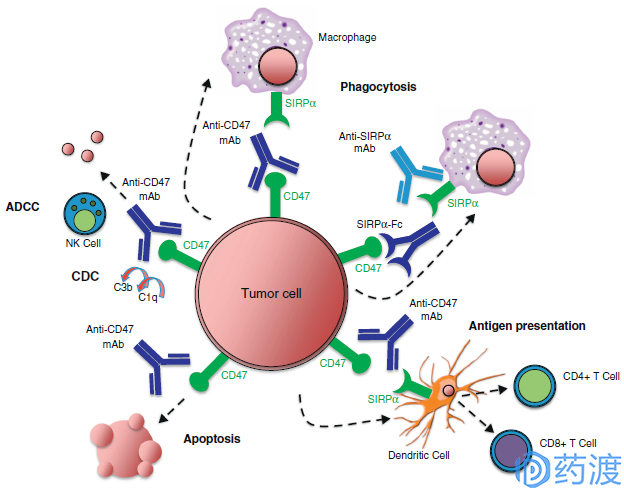
Mechanisms of targeting the CD47–SIRPα pathway in cancer
CD47 and SIRP α
CD47, also known as integrin associated protein (IAP), is a member of the immunoglobulin superfamily. CD47 is widely expressed on the surface of cells and interacts with signal regulatory protein α (SIRP α), thrombospondin-1 (TSP1) and integrins to mediate apoptosis and proliferation. , a series of reactions such as immunization. In 2000, Oldenborg et al. demonstrated that CD47 is an important 'self' marker on the cell surface and an important signal for regulating macrophage phagocytosis. CD47 binds to SIRPα on the surface of macrophages, phosphorylates ITIM, and subsequently recruits SHP-1 protein, producing a cascade of cascades that inhibit macrophage phagocytosis. Tumor cells have a series of programs to evade the human immune system, including secretion of immunosuppressive factors, down-regulation of MHC I expression, and up-regulation of PD-L1 inhibition of CD8+ T cell activity. Different studies have shown that almost all tumor cells and tissues express high levels of CD47, which is three times that of normal cells and tissues.
The three drugs currently in the clinical phase are Huyf7-G4 for Forty Seven, CC-90002 from Celgene (from Inhibrx, code INBRX-103) and TTI-621 from Trillium. It is worth mentioning that the CD47 antibody project of Trillium Therapeutics is a SIRPαFc fusion protein with similar CD47 affinity (nM level) to Hu5F9-G4. SIRPαFc has two major advantages: firstly, its molecular weight is about 80kDa, which has better penetrability and tissue distribution than 150kDa of antibody molecule; secondly, the affinity of SIRPαFc for red blood cells is much lower than that of Hu5F9-G4, indicating It may have better security. In the field of tumor therapy for CD47 antibodies, Trillium Therapeutics' SIRPαFc fusion protein may have a differentiation advantage.
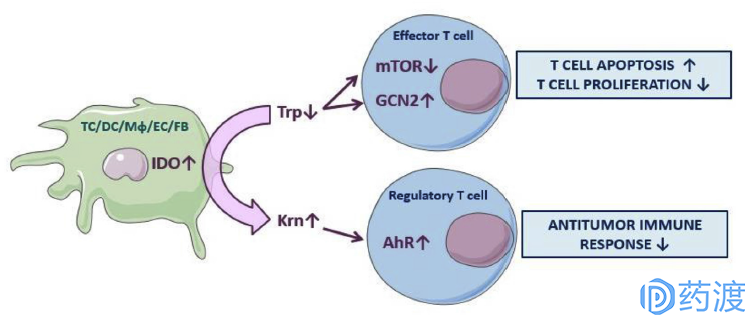
Mechanisms of IDO pathway activity inimmune tolerance
IDO
Indoleamine 2,3-dioxygenase (IDO) consists of 403 amino acid residues and is the only catalyzed epoxidation of tryptophan in cells other than the liver. It is a rate-limiting enzyme that is catabolized along the kynureine pathway (KP). The main function of the human immune system is effector T cells, while Treg Cell (regulatory T cells) plays a negative regulatory role. Therefore, tumor cells are immune to escape, mainly by inhibiting effector T cells and activating Treg Cell. There are three main mechanisms by which IDO regulates immune tolerance: the first mechanism is that the lack of tryptophan inhibits the two signaling pathways of mTORC1 and PKC in effector T cells, resulting in an energy-deficient T cell, growth retardation and Apoptosis. The second mechanism, the shortage of tryptophan, causes a large amount of uncharged tryptophan transfer ribonucleic acid to accumulate in cells, and these unsteady compounds accumulate to activate stress kinases in cells. (GCN2), thereby terminating protein translation and activation of effector T cells. The last mechanism is that kynuric acid, a metabolite produced by the metabolism of tryptophan by IDO, binds to the AHR transcription factor, thereby activating FOXP3 + Treg Cells, which in turn inhibits effector T cells. Activity. The large expression of IDO can help tumor cells to escape immune in the tumor microenvironment. For example, high expression of IDO can inhibit proliferation and maturation of T cells and induce apoptosis, thereby promoting tumor development and the like. Abnormal increases in IDO expression or activity have been shown to be closely related to the pathogenesis of diseases such as depression, Alzheimer's disease, cataracts, and cancer. Therefore, IDO inhibitors may become an effective method for the treatment of these diseases, and have attracted the attention of many scholars and pharmaceutical companies.
There are currently 4 IDO small molecule inhibitors in clinical research, of which epacadostat has registered 20 clinical trials, including a phase III clinical study of melanoma patients. Indoximod developed by NewLink is in clinical phase II research in the United States, and GDC-0919 developed in conjunction with Roche is also in Phase I clinical research in the United States. In addition, BMS and Pfizer IDO inhibitors have also entered the clinical phase I study. On March 24, 2017, Hengrui Pharmaceuticals submitted the SHR9146 clinical registration application to the FDA, which is an oral potent small molecule IDO inhibitor.
Innate immunity - NK cell checkpoint
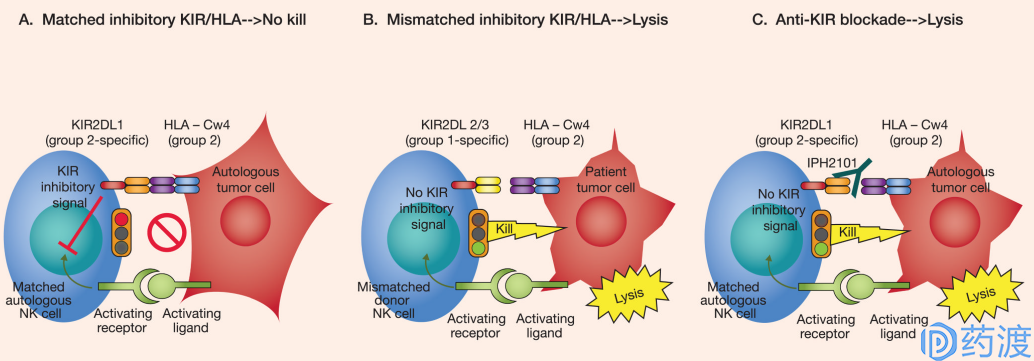
Mechanism of KIR mediated signal
KIR family
The KIR family is a group of highly polymorphic genes that express the surface of most NK cells and some T cells. Some KIR family members (KIR2DL1-3, KIR3DL1 exerts an inhibitory function by binding to MHC molecules (HLA-C/HLA-B). KIR, as a regulator of the NK cell family, has recently become a new target for a variety of tumor immunotherapy. KIR plays a negative regulatory role in NK cell effector function. Inhibition of KIR causes anti-tumor activity of NK cells.
Despite good results in preclinical studies, the results of a phase 1/2 clinical study of KIR2DL1/2/3 monoclonal antibody inhibitor IPH2101 in myeloma patients were not ideal. The KIR2DL1/2/3 mAb inhibitor IPH2102 (lirilumab) is currently being used in combination with drugs targeting PD-1 or CTLA-4 to conduct a phase 1/2 clinical study in patients with advanced solid tumors and hematologic malignancies.
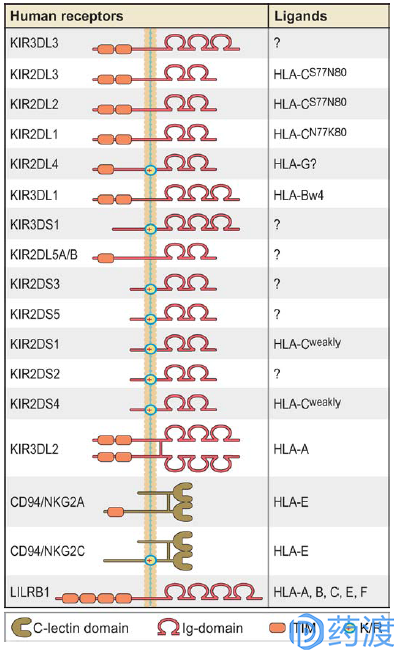
Graphic depiction of human NK cell receptors for MHC class I
CD94/NKG2A
CD94 is present on the surface of NK cells and forms an inhibitory heterodimer with the lectin domain at the C-terminus of NKG2A. It also forms an activated heterodimer with NKG2C or E. T cells can also express CD94/NKG2A receptors, but mainly play a role of inhibitory checkpoints. CD94/NKG2A exerts inhibitory activity by binding to MHC I (HLA-E), and this interaction can be disrupted by targeting ERAP-1. Studies have shown that blocking NKG2A activity can increase antibody-dependent cytotoxicity, and patients with solid tumors overexpressing HLA-E often have a poor prognosis; thus CD94/NKG2A is a promising target in tumor immunotherapy. At present, at least six clinical 1/2 studies of IPH2201 targeting NKG2A are underway, either alone or in combination with PD-1 inhibitors to explore the efficacy of patients with different advanced cancers.
references:
1. Emergingtargets in cancer immunotherapy. Semin Cancer Biol. 2017 Oct 5. pii: S1044-579X(17)30182-7. doi: 10.1016/j.semcancer.2017.10.001.
2. Introduction to checkpoint inhibitors and cancer immunotherapy. Immunol Rev. 2017Mar;276(1):5-8. doi: 10.1111/imr.12531.
3. Combinationcancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov.2015 Aug;14(8):561-84. doi: 10.1038/nrd4591.
4. TargetedTherapy and Checkpoint Immunotherapy Combinations for the Treatment of Cancer. Trends Immunol. 2016 Jul;37(7):462-76. doi: 10.1016/j.it.2016.04.010.
5. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol. 2016 May 20;34:539-73.doi: 10.1146/annurev-immunol-032414-112049.
6. Newcheckpoints in cancer immunotherapy. Immunol Rev. 2017 Mar;276(1):52-65. doi:10.1111/imr.12524.
Youth Biotech CO,. Ltd. , https://www.youtherb.com