Yesterday (July 23), the State Drug Administration approved a total of 6 medical device products, including the high-throughput of Guangzhou Burning Stone Medical Laboratory Co., Ltd. (hereinafter referred to as "burning stone medicine"). The innovative product "Human EGFR/ALK/BRAF/KRAS gene mutation detection kit (reversible end-stop sequencing method)" was registered to obtain the registration of three types of medical device products.
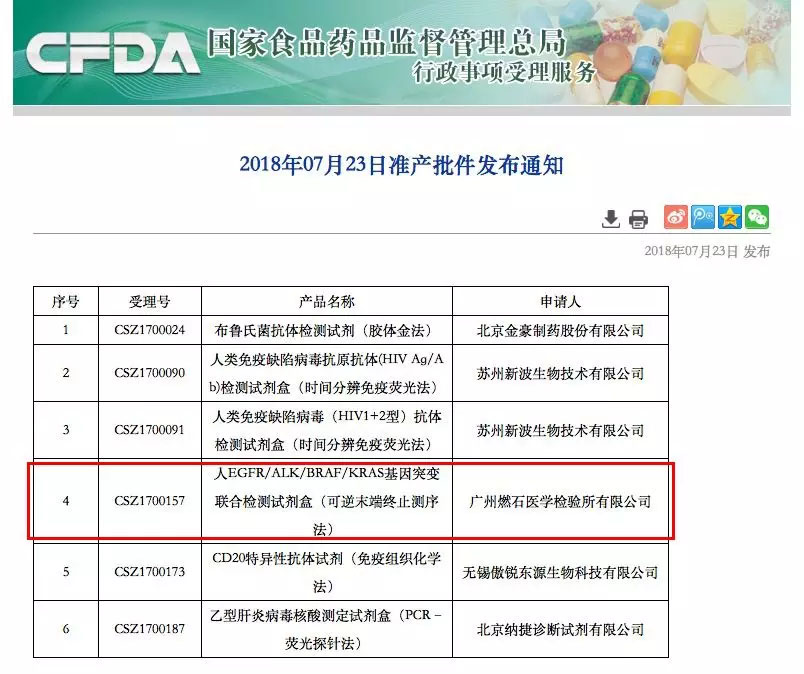
It is understood that the kit is China's first approved multi-gene tumor mutation detection kit based on high-throughput sequencing technology (NGS) and accompanying diagnostic criteria, which will be used to help patients with non-small cell lung cancer to accurately target. The mode of medication. In September 2016, the former State Food and Drug Administration approved the kit as the first tumor NGS testing product in China to enter the “Special Approval Process for Innovative Medical Devicesâ€. After almost two years, after a large number of clinical trials of clinical trials, the State Drug Administration finally issued the “First Certificate of Oncology NGS†through the examination and examination of the registered production quality system.
Compared with traditional genetic testing methods, NGS technology allows patients to simultaneously understand the hotspots and non-hotspot mutations of multiple tumor-related genes in a single test, including point mutations, insertions, and rearrangements (fusion). A variety of variants provide a one-stop detection solution for doctors and patients with multiple targeted drugs, saving test samples and testing time.
The value of NGS in the field of precision medicine and accompanying diagnosis has been widely recognized by clinical experts. Due to the complexity of its technology, the standardization and standardization of NGS quality system has become a bottleneck in its clinical routine. With the approval of the kit for burning stone medicine, the tumor NGS test will be officially entered into the clinical hospital testing department.
The co-founder and COO Yan Shaokun of the Burning Stone Medicine said: The approval of the kit is very significant, which means that we can promote the NGS-based companion diagnosis more quickly and more standardly, and apply it to each NGS testing capability and qualification. Hospitals or third-party testing laboratories to benefit more cancer patients.
Bio Ethanol Fuel,Fuel Ethanol,Ethanol Fuel For Cars,Environment Good Ethanol Fuel
JILIN COFCO BIO-CHEM AND BIO-ENERGY MARKETING CO., LTD , https://www.cofco-biotech.com