Medical Network March 15th Ozanimod is a new drug for the treatment of multiple sclerosis (MS) that is highly regarded by Celgene, which is considered to be a key product affecting the company's future development. However, in recent days, Xinji Medicine received a Refuse to File (RTF) letter, and the FDA refused to conduct a full review of Ozanimod's listing application documents.
What does it mean to receive an RTF letter for a new drug waiting to be marketed? The RTF letter indicates that there are some practical problems with the drugs involved, and in most cases, the new drugs will have to wait a long time to get approval. Even though the delays that seemed to be not a problem were given at the time, it was more common to influence the time limit for approval.
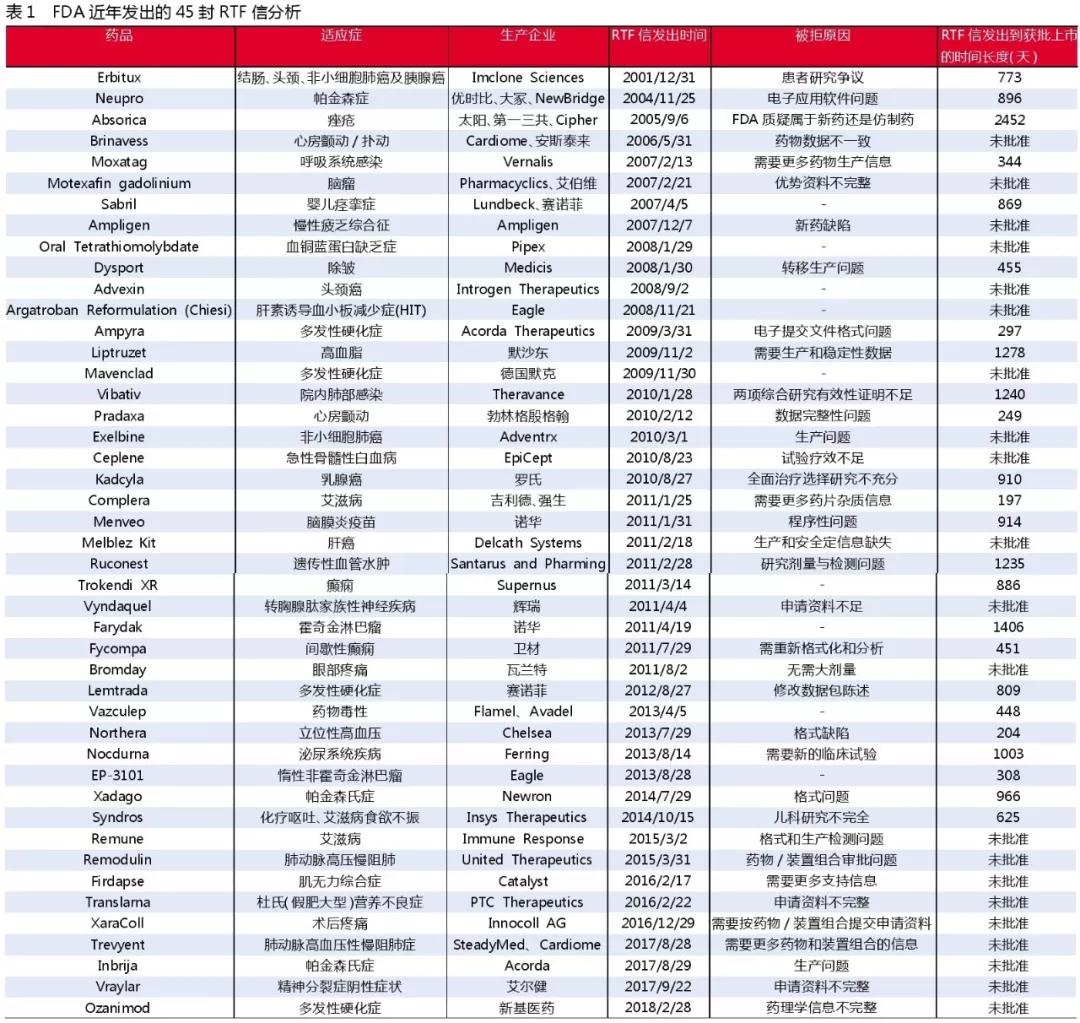
Approved after an average of 800 days
In order to more clearly observe the impact of RTF letters on the launch of new drugs, Forbes columnist Matthew Herper analyzed the RTF letter issued by the FDA since December 31, 2001. The listing of new drugs was delayed or even failed to be listed. The situation has been sorted out.
The extent to which different products are delayed due to RTF letters is not the same. For example, Gilead and Johnson & Johnson's AIDS drug Complera received approval within six months of receiving an FDA's RTF letter for their registration application, essentially without deviating from it. The original process; and Merck's Liptruzet, ezetimibe/atorvastatin, received the RTF letter for the new drug application and waited for three and a half years before being approved.
Since December 31, 2001, a total of 45 RTF letters have been released. Of these, 21 drugs or new-use products involving problems have not been approved, accounting for 47%. The remaining 24 RTF letters ranged from 217 days to nearly 7 years from the time the letter was issued until the drug was approved. For example, the aforementioned Complera compound was newly medicinal for 217 days, and a drug for treating acne, Absorica capsule, was used for nearly 7 years.
The average duration was 800 days, of which 14 drugs were not approved within two years, accounting for 58%. In addition, none of the applications for drugs and new drug indications received from the RTF letter since 2015 have been approved.
Analysis of 45 RTF letters issued by the FDA in recent years
New base MS new drugs every second counts
Under what circumstances can the approval delay be shortened? In five cases where the approval time was less than one year, the FDA considered that the applied drug had an important effect on the treatment of the disease , which was sufficient to justify its priority review. The other two issues related to the format of the application.
New Base Medicine has not yet given an exact response to Ozanimod's related questions. Umer Raffat, an analyst at investment bank Evercore/ISI, speculates that the rejection of a listing application may be related to the metabolites of the drug —that is, the FDA wants to specify how the drug breaks down into other chemicals in the body. It will not be clear how long these problems will take to resolve them.
But for Xinji Medicine , time is very important. Gilenya is Novartis's oral capsule for the treatment of MS. Its patent will expire on February 18, 2019. The related generic drugs are expected to be temporarily approved this year. Brian Skorny, an analyst at Baird Investment Bank, is concerned that the pace of new-based medicines behind the Fingalmod generics will weaken the sales potential of ozanimod. Both analysts said that the most important issue facing the company was to negotiate with generic companies to delay the competitive pressure of its popular anticancer drug, Revlimid.
White Sesame Oil,Ground Sesame Seed Oil,White Sesame Seed Oil,A Grade Sesame Oil
Chinese Seasoning (Shandong) Trading Co.,Ltd , https://www.zt-trading.com