Pfizer's original, trade name is Svo's linezolid, an oxazolidinone antibiotic. Swowo received FDA approval in 2000. At present, the domestic generic drug preparations are mainly from the following companies: Jiangsu Haosen, Jiangsu Zhengda Fenghai, Zhejiang Medicine, Zhengda Tianqing, Sichuan Meida Kangjiale Pharmaceutical.
It can be seen from the existing literature that the step of synthesizing linezolid has at least 7 steps, and some synthetic routes contain a group protection and deprotection process. It takes more than 60 hours from the starting material to the final product, and the overall process takes a long time, the operation is cumbersome, and the efficiency is low.
Let's take a look at this masterpiece. Interested friends can download the full text to read DOI: 10.1002 / anie. 201901814.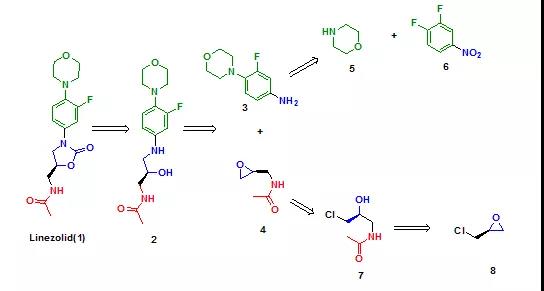
The authors decomposed the linezolid synthesis pathway into six compounds by a classical inverse synthesis analysis strategy (Fig. 1).
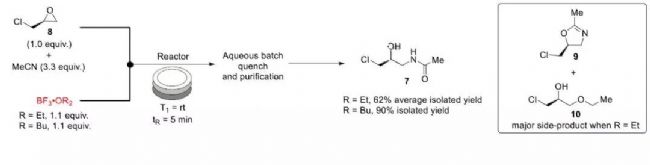
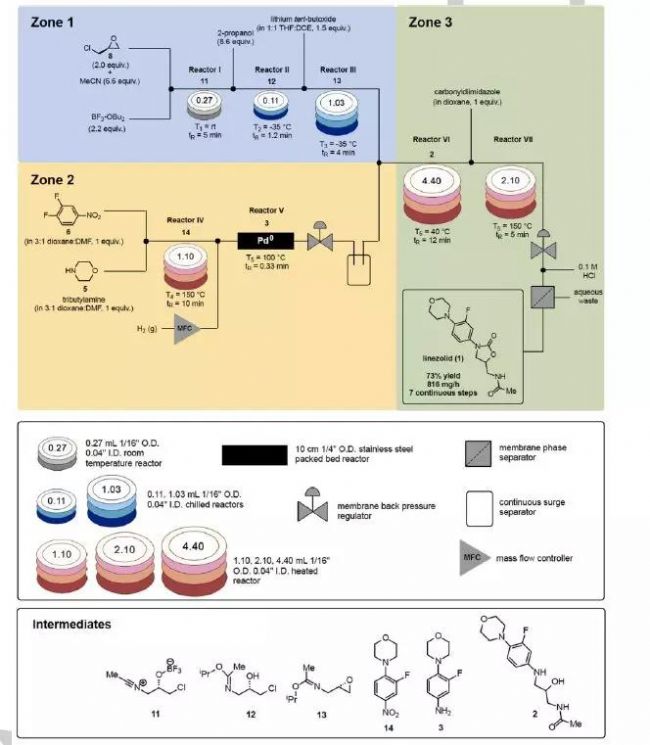 Figure 3: Seven-step synthesis of linezolid
Figure 3: Seven-step synthesis of linezolid
Experimental summary:
It can be seen from the existing literature that the step of synthesizing linezolid has at least 7 steps, and some synthetic routes contain a group protection and deprotection process. It takes more than 60 hours from the starting material to the final product, and the overall process takes a long time, the operation is cumbersome, and the efficiency is low.
Let's take a look at this masterpiece. Interested friends can download the full text to read DOI: 10.1002 / anie. 201901814.

Figure 1: Inverse Synthesis Analysis Strategy
The authors decomposed the linezolid synthesis pathway into six compounds by a classical inverse synthesis analysis strategy (Fig. 1).
Starting from the initial starting material, study the entire synthetic route. First, the authors started from the synthesis of compound 7 and prepared the amide 7 in one step by Ritter reaction.
In the preparation process, it was not smooth. In the process of using BF3·C2H5OC2H5, a large amount of by-products were detected in the reaction, and the main product yield was low. Under such circumstances, the author carefully studied the reaction mechanism and tried to use BF3. • C2H5OC2H5 was replaced by C8H18BF3O, and finally compound 7 was obtained in 90% yield (Fig. 2).

Figure 2: Route optimization of compound 7 in a continuous flow process
On the basis of the synthesis of compound 7, the authors began a continuous flow process study for each step. Finally, the authors perfectly combined the reaction and quenching in a continuous flow process with no separation and purification steps in between, directly synthesizing API-linezolidamide from the starting material (Figure 3).
 Figure 3: Seven-step synthesis of linezolid
Figure 3: Seven-step synthesis of linezolid Finally, the authors purified the product and obtained the final product with a total yield of 73%. The flux was about 816 mg/h. The overall reaction time was shortened from 60 hours in the kettle to 27 minutes. Through calculation, the author feels that the process has potential industrial application prospects.
Experimental summary:
The publication of this document indicates that the full continuous flow process has a good application value in the synthesis of drug substances. The use of continuous flow technology to synthesize linezolid has a lot of research work to be truly industrialized, but this document brings more hope for the preparation of APIs by continuous flow technology, and also brings research power to researchers. .
Two-Piece Baseplate,2 Piece Colostomy Bags Chassis,Ostomy Bags Chassis,Hypoallergenic Ostomy Chassis
Wenzhou Celecare Medical Instruments Co.,Ltd , https://www.celecaremed.com