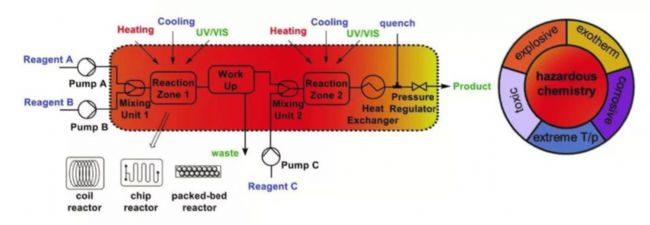
With the innovation of technology, microchannel reactors have revolutionized chemical equipment. The microreactor has a high specific surface area and a small internal volume, making it possible to carry out reactions that are not smoothly achieved in conventional batch reactors. In addition, the previously unrealistic ("forgotten chemistry") or almost impossible ("forgotten chemistry") orvirtually impossible ("forbidden chemistry") operation risk on the microreactor is greatly reduced small. Therefore, the application of continuous flow microchannel reactors in industrialization has gradually gained industry recognition.
Professor Oliver Kappe is a well-known continuous flow expert in Europe. Bernhard Gutmann and Oliver Kappe have selected some cases from Zui's recent literature. These are previously unrealistic ("forgotten chemistry") or almost impossible. The use of chemical reactions ("prohibited chemistry") in continuous flow microreactors is discussed.
Some concepts of flow chemistry in microreactors:
1) Reactive reagents can change rapidly along the reaction path at precisely specified points and conditions
2) Unit operations can be integrated into a fully continuous production line
3) Because of its great heat transfer and mass transfer ability, the reaction can be operated under high pressure and high temperature conditions by strengthening the reaction conditions. One of the zui's remarkable features is the very high specific surface area. The specific surface area of ​​a microreactor is typically increased by two orders of magnitude relative to a conventional kettle reactor. The high specific surface area gives the reactor a strong heat exchange capacity, and can be rapidly heated or cooled to the temperature required for the reaction to suppress the formation of hot spots.
In addition, due to the small size, the mass transfer efficiency of the microreactor is also greatly improved. Thus, with a strong exothermic reaction, the reaction can be achieved quickly and safely in the microreactor. Reactions that have previously been considered difficult or impossible to complete have become controllable and less risky in microreactors.
 Photocatalytic continuous synthesis of cinnabarin is no stranger to artemisinin. Artemisinin antimalarial drugs, usually extracted from the plant Artemisia annua, are far from meeting the annual demand for the drug. The chemical synthesis of artemisinin is of great significance.
Photocatalytic continuous synthesis of cinnabarin is no stranger to artemisinin. Artemisinin antimalarial drugs, usually extracted from the plant Artemisia annua, are far from meeting the annual demand for the drug. The chemical synthesis of artemisinin is of great significance.
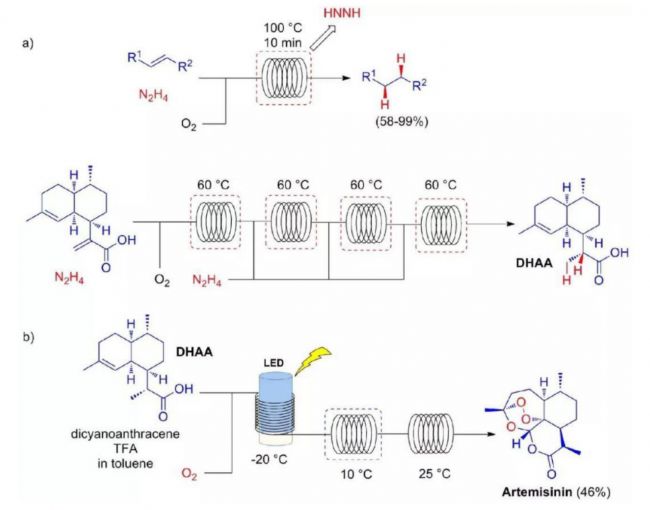 Figure 1. In a continuous flow reactor, multi-step reaction and purification steps can be integrated into a single continuous system to reduce the transport or storage of unstable intermediates.
Figure 1. In a continuous flow reactor, multi-step reaction and purification steps can be integrated into a single continuous system to reduce the transport or storage of unstable intermediates.
Dihydroartemisinic acid (DHAA) is a semi-synthetic raw material for artemisinin. The key step in the current semi-synthetic artemisinin is the reaction of singlet with DHAA ene. The reaction follows the cleavage of the oxygen-oxygen bonds and the subsequent addition of triplet oxygen. This triggers the subsequent condensation reaction to form the zui final product (Scheme b). The entire reaction process was carried out by the German chemist Max Planck at the Institute of Colloid and Interface. As a completely continuous chemical process, DHAA, a photosensitizer and an acid catalyst are mixed with oxygen through a continuous reactor. After the photochemical step, the mixture is heated in the microreactor to complete the oxygen-oxygen bond cleavage and subsequent oxidation. Artemisinin is obtained by the triplet oxygen and the subsequent condensation.
Singlet oxygen as a cheap and green reagent has a very high application value in contemporary organic synthesis. In the conventional batch reactor, since the photochemical reaction itself is difficult to scale, the industrial application of such a reaction is limited. In addition, the high reactivity and short life of singlet oxygen can cause serious safety problems, which also brings technical challenges to experiments and production. The advent of photochemical microreactors has made the application of commercial-scale photochemical reactions shine. It is worth mentioning that Corning reactor technology has extremely high application value in this field, especially photochemical reactors, which integrate the continuous flow of Corning reactors.
Efficient heat transfer, high efficiency mass transfer, excellent light transmission performance, high light intensity, small test and industrial scale-up production without amplification effect, will bring new development of artemisinin and dihydroartemisinin synthesis and industrialization. Breakthrough. Nitrification and hydrogenation reactions are common chemical reactions and a problem that plagues chemical companies. Most of the nitrification and hydrogenation reactions have large heat release, fast reaction speed and difficult process control. In recent years, cases of accidents involving nitrification have often been reported. Finding effective solutions is imminent.
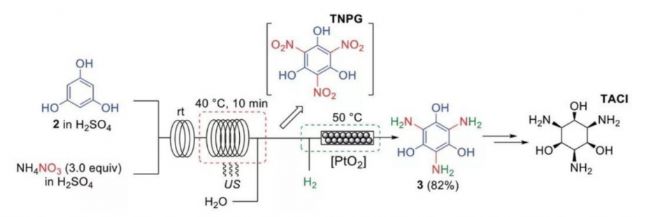 Figure 2. The multi-step nitrification microreactor technology for continuous flow of electron-rich starting materials gives a better solution. Even the multi-step nitration of the electron-rich starting materials shown above can be achieved smoothly in the microreactor.
Figure 2. The multi-step nitrification microreactor technology for continuous flow of electron-rich starting materials gives a better solution. Even the multi-step nitration of the electron-rich starting materials shown above can be achieved smoothly in the microreactor.
Azide reaction microreactors not only provide an effective means to control fast or exothermic reactions, but researchers use the extreme process conditions of microreactors to explore "new process windows."
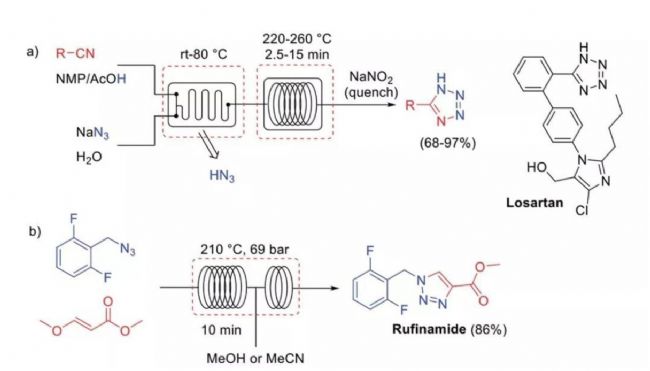
![]() Figure 3. High-intensification process The azide reagent has been successfully summarized in a continuous flow microreactor:
Figure 3. High-intensification process The azide reagent has been successfully summarized in a continuous flow microreactor:
l The chemical industry needs to use energy and materials more efficiently with environmental and economic pressures. Process enhancement is very important in the chemical synthesis of new reagents. As technology advances, the use of new devices, new synthetic paradigms become accessible.
l From the traditional batch operation to the continuous reaction in the microreactor, the chemical safety operating range can be greatly expanded. The reaction can achieve reactions (such as high concentrations, high temperatures, and/or pressures) that have hitherto been unfeasible in microstructure devices.
l In addition, reactions involving unstable, explosive or other hazardous intermediates are relatively safe and easy to operate using microreactors, enabling large-scale safe production.
l zui short and zui elegant synthesis of a molecule often requires the use of hazardous reagents or challenging process conditions, and continuous flow microreactors provide a means to develop these chemicals and maximize their potential.

Professor Oliver Kappe is a well-known continuous flow expert in Europe. Bernhard Gutmann and Oliver Kappe have selected some cases from Zui's recent literature. These are previously unrealistic ("forgotten chemistry") or almost impossible. The use of chemical reactions ("prohibited chemistry") in continuous flow microreactors is discussed.
Some concepts of flow chemistry in microreactors:
1) Reactive reagents can change rapidly along the reaction path at precisely specified points and conditions
2) Unit operations can be integrated into a fully continuous production line
3) Because of its great heat transfer and mass transfer ability, the reaction can be operated under high pressure and high temperature conditions by strengthening the reaction conditions. One of the zui's remarkable features is the very high specific surface area. The specific surface area of ​​a microreactor is typically increased by two orders of magnitude relative to a conventional kettle reactor. The high specific surface area gives the reactor a strong heat exchange capacity, and can be rapidly heated or cooled to the temperature required for the reaction to suppress the formation of hot spots.
In addition, due to the small size, the mass transfer efficiency of the microreactor is also greatly improved. Thus, with a strong exothermic reaction, the reaction can be achieved quickly and safely in the microreactor. Reactions that have previously been considered difficult or impossible to complete have become controllable and less risky in microreactors.
 Photocatalytic continuous synthesis of cinnabarin is no stranger to artemisinin. Artemisinin antimalarial drugs, usually extracted from the plant Artemisia annua, are far from meeting the annual demand for the drug. The chemical synthesis of artemisinin is of great significance.
Photocatalytic continuous synthesis of cinnabarin is no stranger to artemisinin. Artemisinin antimalarial drugs, usually extracted from the plant Artemisia annua, are far from meeting the annual demand for the drug. The chemical synthesis of artemisinin is of great significance.  Figure 1. In a continuous flow reactor, multi-step reaction and purification steps can be integrated into a single continuous system to reduce the transport or storage of unstable intermediates.
Figure 1. In a continuous flow reactor, multi-step reaction and purification steps can be integrated into a single continuous system to reduce the transport or storage of unstable intermediates. Dihydroartemisinic acid (DHAA) is a semi-synthetic raw material for artemisinin. The key step in the current semi-synthetic artemisinin is the reaction of singlet with DHAA ene. The reaction follows the cleavage of the oxygen-oxygen bonds and the subsequent addition of triplet oxygen. This triggers the subsequent condensation reaction to form the zui final product (Scheme b). The entire reaction process was carried out by the German chemist Max Planck at the Institute of Colloid and Interface. As a completely continuous chemical process, DHAA, a photosensitizer and an acid catalyst are mixed with oxygen through a continuous reactor. After the photochemical step, the mixture is heated in the microreactor to complete the oxygen-oxygen bond cleavage and subsequent oxidation. Artemisinin is obtained by the triplet oxygen and the subsequent condensation.
Singlet oxygen as a cheap and green reagent has a very high application value in contemporary organic synthesis. In the conventional batch reactor, since the photochemical reaction itself is difficult to scale, the industrial application of such a reaction is limited. In addition, the high reactivity and short life of singlet oxygen can cause serious safety problems, which also brings technical challenges to experiments and production. The advent of photochemical microreactors has made the application of commercial-scale photochemical reactions shine. It is worth mentioning that Corning reactor technology has extremely high application value in this field, especially photochemical reactors, which integrate the continuous flow of Corning reactors.
Efficient heat transfer, high efficiency mass transfer, excellent light transmission performance, high light intensity, small test and industrial scale-up production without amplification effect, will bring new development of artemisinin and dihydroartemisinin synthesis and industrialization. Breakthrough. Nitrification and hydrogenation reactions are common chemical reactions and a problem that plagues chemical companies. Most of the nitrification and hydrogenation reactions have large heat release, fast reaction speed and difficult process control. In recent years, cases of accidents involving nitrification have often been reported. Finding effective solutions is imminent.
 Figure 2. The multi-step nitrification microreactor technology for continuous flow of electron-rich starting materials gives a better solution. Even the multi-step nitration of the electron-rich starting materials shown above can be achieved smoothly in the microreactor.
Figure 2. The multi-step nitrification microreactor technology for continuous flow of electron-rich starting materials gives a better solution. Even the multi-step nitration of the electron-rich starting materials shown above can be achieved smoothly in the microreactor. Azide reaction microreactors not only provide an effective means to control fast or exothermic reactions, but researchers use the extreme process conditions of microreactors to explore "new process windows."

l The chemical industry needs to use energy and materials more efficiently with environmental and economic pressures. Process enhancement is very important in the chemical synthesis of new reagents. As technology advances, the use of new devices, new synthetic paradigms become accessible.
l From the traditional batch operation to the continuous reaction in the microreactor, the chemical safety operating range can be greatly expanded. The reaction can achieve reactions (such as high concentrations, high temperatures, and/or pressures) that have hitherto been unfeasible in microstructure devices.
l In addition, reactions involving unstable, explosive or other hazardous intermediates are relatively safe and easy to operate using microreactors, enabling large-scale safe production.
l zui short and zui elegant synthesis of a molecule often requires the use of hazardous reagents or challenging process conditions, and continuous flow microreactors provide a means to develop these chemicals and maximize their potential.

Hot Sell Amazon USA & UK pH Test Strips, pH paper 0-14
Advantages:
1,Imported raw materials from German to stable product quality.
2,We uphold integrity and strict quality control, and the export rate of return of zero. (peers 3.5%)
3, Lot number, Manufacture date , Use by/Expiry date are printed clearly on the box and label.
4,We have a system for recording production batch Numbers and tracking product after-sales problems.
Water Analysis Kit,Water Ph Test Strips,Water Purity Test Kit,Digital Aquarium Water Tester
Changchun LYZ Technology Co., Ltd , https://www.lyzstrips.com