Medical Network April 2nd, March 29th, CDE official website publicized the latest batch of products to be reviewed first, with 8 acceptance numbers, 4 varieties (according to variety name + reporting enterprise) to be included in the priority review, 4 All varieties are listed in the generic category 3, and are intended to be included in the “first production line production, which has been listed in the USâ€. It is intended to be included in the priority review.
Table 1: Varieties to be included in the priority review
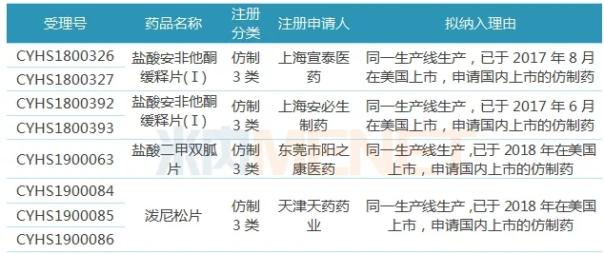
(Source: CDE official website, Mene network database)
Figure 1: Competitive pattern of bupropion hydrochloride sustained-release tablets (I)

(Source: Minenet one-click search)
At present, there is no bupropion hydrochloride sustained-release tablet (I) on the market in China. The original manufacturer GlaxoSmithKline is applying for import. The import application was accepted by CDE on May 9, 2018. In the status of review and approval, Shanghai Xuantai Pharmaceutical and Shanghai Ambition Pharmaceuticals have submitted three types of generic drug companies for imitation, and are currently in the state of “in the review and approval (in the drug trial center)â€.
Figure 2: Sales of metformin hydrochloride tablets in Chinese public medical institutions in 2013-2017 (unit: 10,000 yuan)
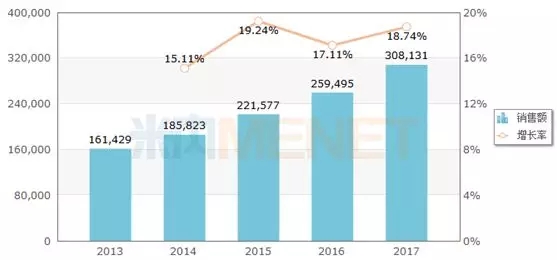
(Source: Minernet China's public medical institutions terminal competition pattern)
According to the data from the intranet, the sales of metformin hydrochloride in China's public medical institutions in 2017 was 3.081 billion yuan, an increase of 18.74% compared with last year. The original researcher Squibb occupied more than half of the market. The submerged dosage forms of metformin hydrochloride currently on the market include ordinary tablets, sustained-release tablets, enteric-coated tablets, etc. Among them, there are 114 manufacturers that have approved the production of metformin hydrochloride tablets.
Figure 3: Application for consistency evaluation of metformin hydrochloride tablets

(Source: Minenet one-click search)
According to the new registration classification, there are 2 enterprises submitting the application for metformin hydrochloride. They are Dongyang Pharmaceutical Co., Ltd. Dongguan Yangzhikang Medicine and Guangdong Saikang Pharmaceutical Factory. The products of Dongguan Yangzhikang Medicine are expected to be transferred to China through foreign countries. The way of priority review speeds up the listing, which is regarded as passing the consistency evaluation.
There are 25 companies submitting applications according to the consistency evaluation supplementary application. The products of 4 enterprises have passed or agreed to pass the consistency evaluation. Among them, the metformin hydrochloride tablets of Shijiazhuang Ouyi Pharmaceutical Co., Ltd. were listed and approved according to the generic 6 categories. Production, the first review.
Source: Minenet database, CDE official website
Corn Gluten Meal Lawn,Harmful Monosodium Glutamate,Dog Corn Gluten Meal,New Edible Starchy Tuber
JILIN COFCO BIO-CHEM AND BIO-ENERGY MARKETING CO., LTD , https://www.cofco-biotech.com