In the past few years, the approval of PD-1, PD-L1, and CTLA4 antibody drugs has stimulated the global development of monoclonal antibody drugs for immune checkpoints. With the deepening of tumor immunology research, immunotherapy has entered a new stage. Due to its unique advantages, Bispecific antibody (BsAb) has gradually occupied a place in the drug research and development stage. Immune escape is often accompanied by a variety of different or overlapping mechanisms, and a single antibody binds only to a specific target, making the treatment effect compromised. As an engineered antibody, BsAb can simultaneously bind two epitopes, block or activate dual-target signaling pathways, mediate immune cells to better kill tumor cells, and thus may be linked to single antibodies or even antibodies. Use better clinical treatment results.
According to incomplete statistics, nearly 40 units at home and abroad have been deployed at the immunological checkpoint bispecific antibody, and 10 immunological checkpoint bispecific antibodies are in clinical stage. Popular research targets are mainly concentrated in CTLA4/PD-1/PD- L1/OX40/4-1BB/CD40.
The following are the progress of bispecific antibodies associated with each target:
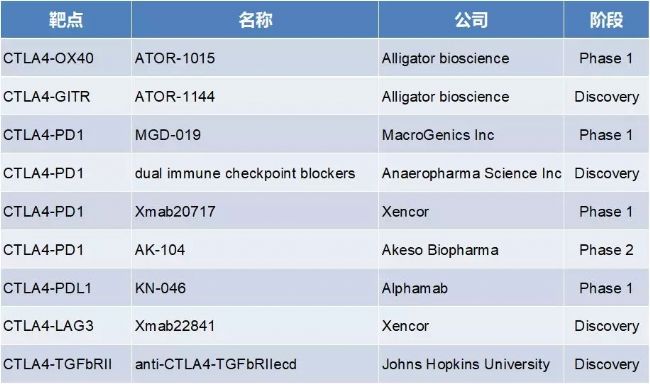
1. CTLA4 target-related BsAb
There are 5 antibodies in the CTLA4 target-associated BsAb in the clinical stage I/II. The domestic research and development of Kangfang Bio and Corning Jerry are faster and take the lead. A total of 9 anti-drugs were developed at this target, 4 of which were CTLA4-PD-1 BsAb.
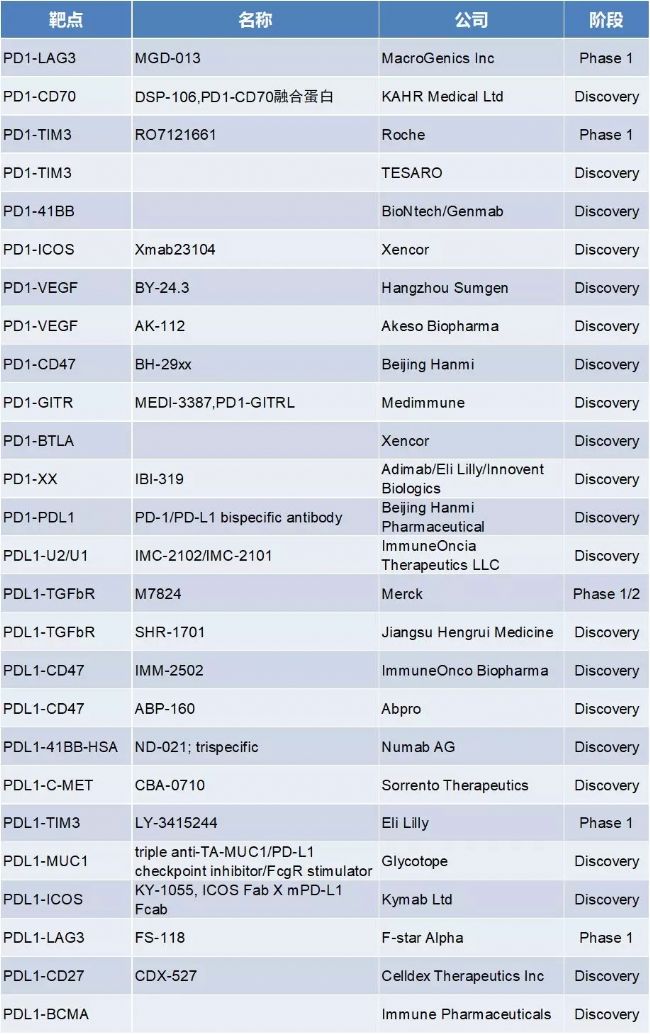
2. PD-1/PD-L1 target-related BsAb
More than 30 BsAbs related to PD-1 and PD-L1 target development have become popular targets. The target combined with PD1 has the most PD1-CTLA4, including other LAG3, TIM3, CD47, BTLA inhibitory targets, and OX40, 41BB, ICOS, GITR, and CD27 activating target combinations . Domestic pharmaceutical companies such as Hangzhou Shangjian, Kangfang Bio, Beijing Hanmei Pharmaceutical, Cinda Bio, Jiangsu Hengrui, and Yiming Anke have their layouts. They are currently in the preclinical research stage and have not obtained clinical approvals.
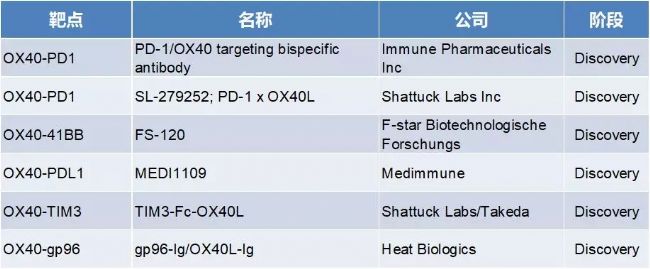
3. OX40 target related BsAb
Six OX40 target-related BsAbs were in the development phase, of which about 50% were combined with PD-1/PD-L1.
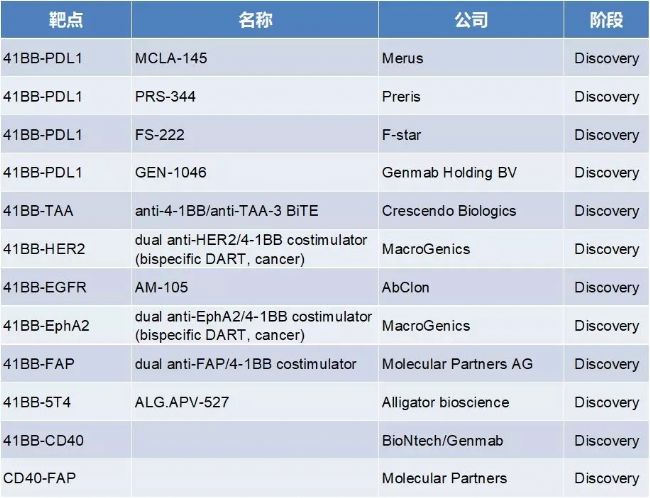
4. 4-1BB and CD40 target related BsAb
The 4-1BB-PD-L1 target BsAb is the most combined form and is currently in the preclinical development stage.
From the above data, BsAb development is developing rapidly, and the research and development speed of domestic and foreign pharmaceutical companies is gradually shortening, and it has strong research and development potential in the field of immunotherapy. However, most BsAbs are still in the pre-clinical development stage, and there are still many challenges from the industrialization of the market. It is expected that more effective antibody treatments will be obtained in the near future to treat various cancers and self-indications.
Bio-Science has the world's most complete and stable immunological checkpoints for humanized mice and a series of dual-sourced mouse platforms with complete targets. The in vivo efficacy evaluation of bispecific antibodies was performed for a number of companies. We hope to add a little boost to the speed of pre-clinical development of bispecific antibodies.
Bio-Symbol B-hCTLA4/hOX40 double-sourced mice

B-hCTLA4/hOX40 double-directed mice for antibody-linked drug efficacy experiment
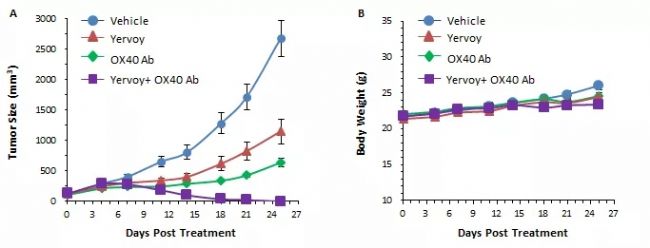
Fig. 1 Mouse colon cancer cell line MC38 was transplanted subcutaneously into B-hCTLA4/hOX40 homozygous mice, and animals were enrolled into control group and treatment group (n=8) when the tumor volume was about 150±50 mm 3 .
The results showed that the combination of anti-CTLA4 antibody (Yervoy) and anti-human OX40 antibody showed more obvious tumor inhibition than the drug alone group, which proved that B-hCTLA4/hOX40 mice were used to evaluate the combination of CTLA4 and OX40 antibodies. A powerful tool . A. Mean tumor volume ± SEM, B. Mouse mean body weight ± SEM.
Reference materials:
Cortellis database
Beck, A., Wurch, T., Bailly, C., & Corvaia, N. (2010). Strategies and challenges for the next generation of therapeutic antibodies. Nature Reviews Immunology, 10(5), 345–352.

Scan code to pay attention to the 100 Olympics map to learn more about consulting
dental chair accessories,chair accessories parts dental,dental chair spare parts,dental chair parts
Foshan Ja Suo Medical Device Co., LTD , https://www.jasuodental.com