Part 3 China Medical Device Regulations, Administrative Charges and Approval Results
3.1 Overview of China's Medical Device Regulations in 2016
Related reading: The most comprehensive 2016 medical equipment industry blue book in history is released (on)
In 2016, China's medical device industry regulations have gradually improved. In this year, the "Quality Management Regulations for Clinical Devices Clinical Trials" was promulgated, which provided better regulatory guarantees for the development of medical device clinical trials. At the same time, the publication of a number of working papers, important documents, guiding principles and solicitation of opinions has raised the standard and supervision of the entire industry and laid a solid foundation for the healthy development of the entire medical device industry. As the leading provider of medical device clinical trial CRO and medical device integrated services, Aostar can provide one-stop overall regulatory solutions for medical device companies. The following is an overview of the release of China's medical device regulations in 2016.
.jpg)
3.2 Overview of Medical Device Registration Fees in 2016
Medical device product registration is designed to obtain a legal listing pass through the market access approval of the regulatory authorities. The standards for registration fees for medical devices vary from country to country. As a high-end medical device clinical registration specialist, Austin summarizes the details of global medical device registration fees and provides a reference for your medical device investment.
3.2.1 US FDA medical device registration fee
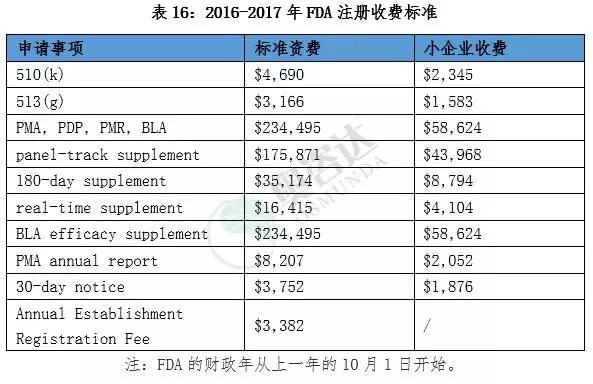
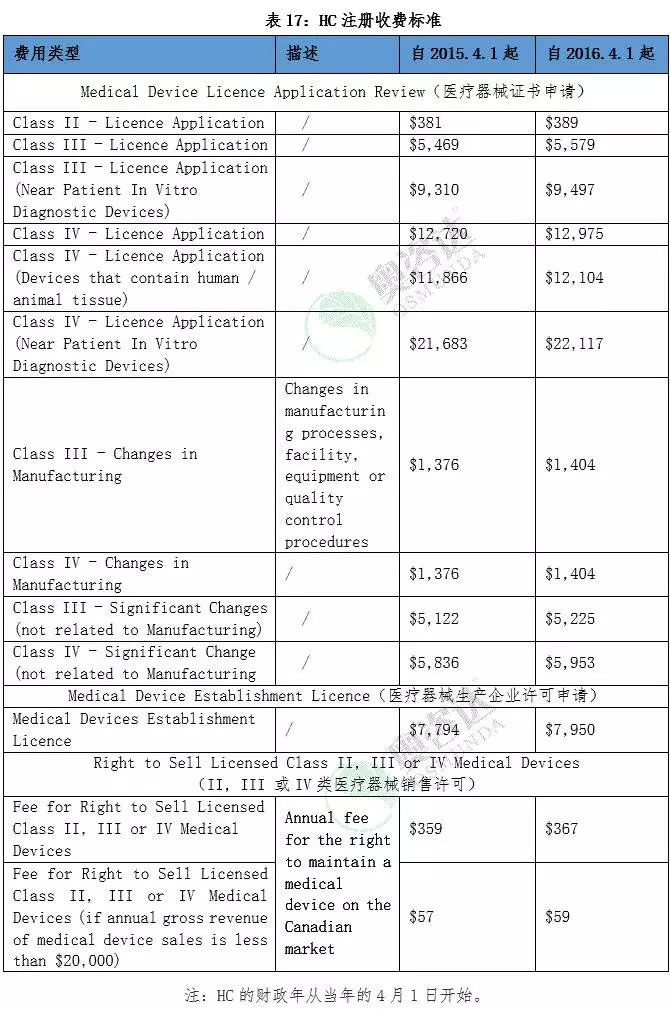
3.2.2. Canadian HC medical device registration fee
China Extract Powder For Use As Dietary Supplement Extract Powder, Extract Powder Manufacturer
Shaanxi Kang New Pharmaceutical co., Ltd. , https://www.bio-pharmacies.com